HEAT 10 1 TEMPERATURE AND THERMAL EQUILIBRIUM DEFINING

HEAT
10. 1 TEMPERATURE AND THERMAL EQUILIBRIUM DEFINING TEMPERATURE Temperature is proportional to the kinetic energy of atoms and molecules. • Therefore, temperature is a measure of kinetic energy. v Heat (Internal Energy) v • Internal energy – the energy of a substance due to the random motions of its component particles (total energy of the particles) • Our touch is a qualitative indicator of temperature The sensation of hot or cold depends on the temperature of the skin, which can be misleading. • http: //www. youtube. com/watch? v=vq. Db. MEd. Li. Cs
Energy must be either added or removed from a substance to change its temperature. v Thermal equilibrium is the state in which two bodies in physical contact with each other have identical temperatures. §Temperature is only meaningful when it is stable. §Thermal equilibrium is the basis for measuring temperature with a thermometer.
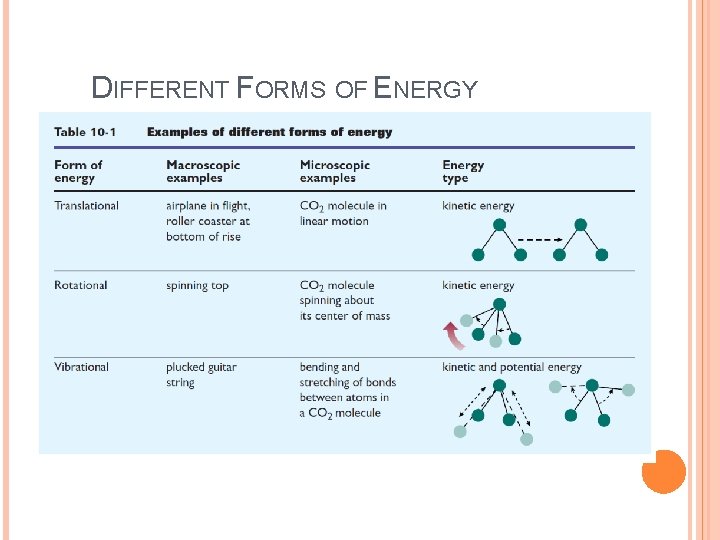
DIFFERENT FORMS OF ENERGY
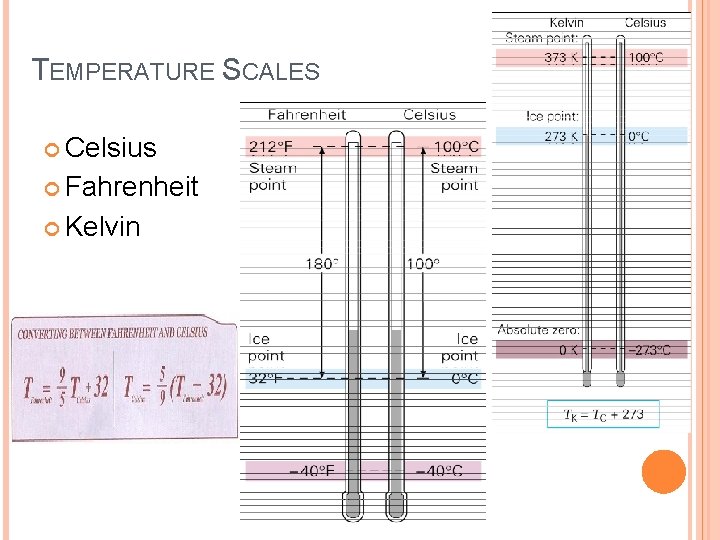
TEMPERATURE SCALES Celsius Fahrenheit Kelvin
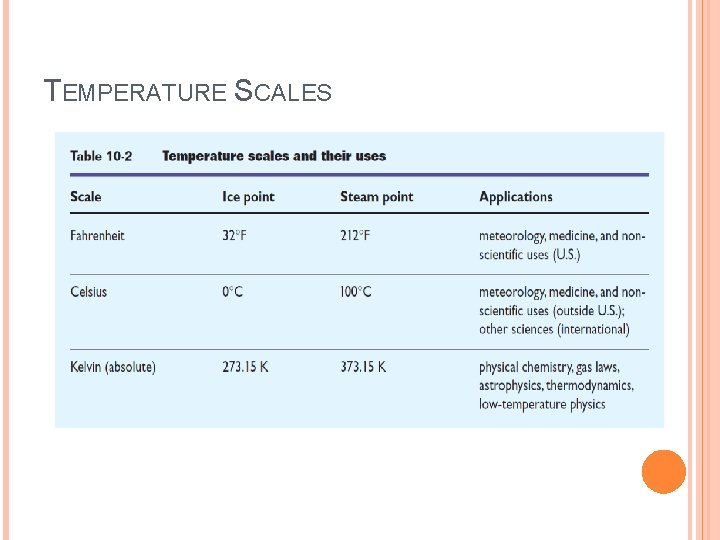
TEMPERATURE SCALES
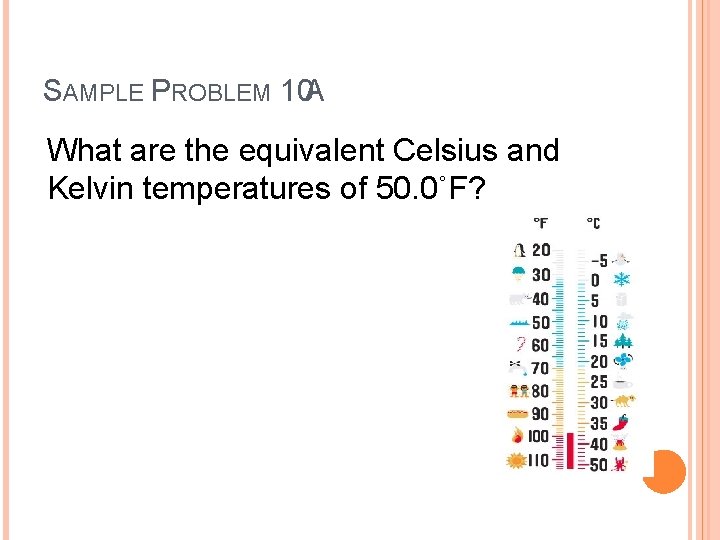
SAMPLE PROBLEM 10 A What are the equivalent Celsius and Kelvin temperatures of 50. 0˚F?
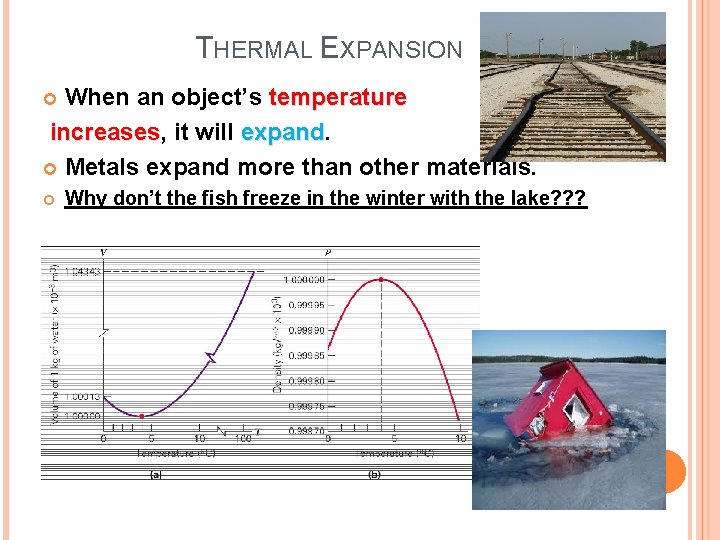
THERMAL EXPANSION When an object’s temperature increases, increases it will expand Metals expand more than other materials. Why don’t the fish freeze in the winter with the lake? ? ?
- Slides: 8