HCV update Ardis Ann Moe UCLA CARE clinicNEVHC














































- Slides: 53

HCV update Ardis Ann Moe UCLA CARE clinic/NEVHC Van Nuys 21 June 2014 amoe@mednet. ucla. edu

Goals: � 1) terminology of hep C � 2) benefits of hep C treatment � 3)drug interaction issues with HIV meds �My thanks to my colleague Debika Bhattacharya for use of her slides for this presentation.

Terminology

� Hep load C viral load is not the same as HIV viral ◦ Hep C viral load does not correlate with risk of death, cirrhosis, liver damage.

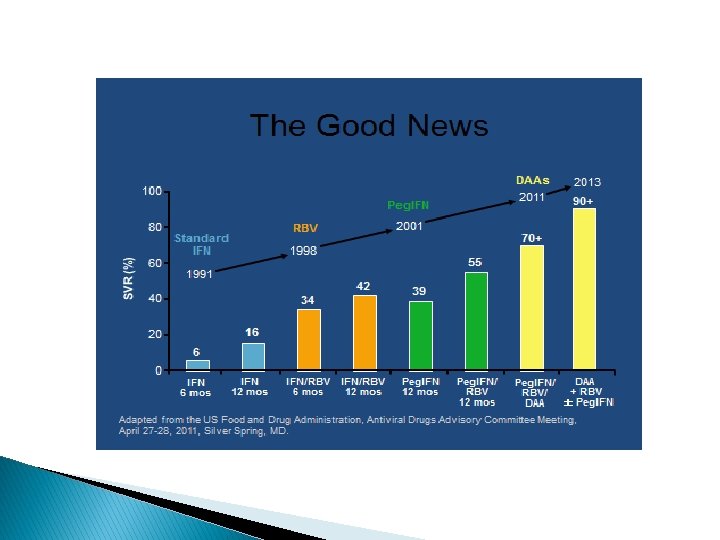
� Hep C can be cured with current medications, unlike HIV. ◦ Cure=SVR “sustained virological response” ◦ Hep C viral load 6 months after completing treatment is undetectable = SVR

� CHILD score: A, B or C. (also scored numerically: 5 or more points) ◦ Risk of death from cirrhosis. ◦ Only to be used in patients with documented cirrhosis ◦ Important since simeprevir contraindicated in patients with CHILD score>5. (or B or C) ◦ Website for calculator for CHILD score: ◦ : http: //www. mdcalc. com/child-pugh-score-forcirrhosis-mortality/ �

Benefits of Hep C Treatment

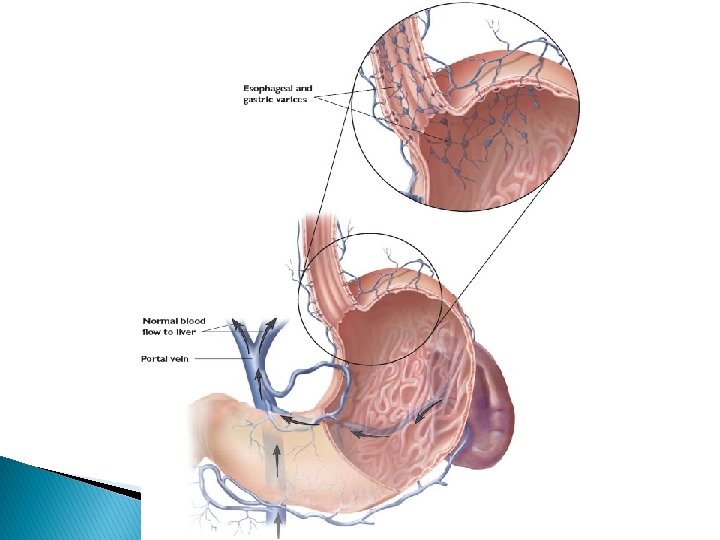
� Patients who do develop cirrhosis have many problems: ◦ ◦ ◦ ◦ Esophageal varices and recurrent internal bleeding Ascites (fluid on the abdomen) Brain damage from hepatic encephalopathy liver cancer Problems with medications Leg edema Jaundice





� Even without cirrhosis, there are complications of hep C ◦ ◦ Fatigue Cryoglobulimia (kidney damage) Porphyria cutanea tarda Increased risk of diabetes



� Treatment of hep C reduces or eliminates risk of liver cancer, cirrhosis, cryoglobulinia, and porphyria. � However, cirrhosis is permanent scarring, so once there is cirrhosis, there is always some risk of liver cancer in the future, even if hep C cured. � Treatment of hepatitis C also appears to alleviate fatigue. Hepatology. 2014 Apr 5


Treatment issues with HIV



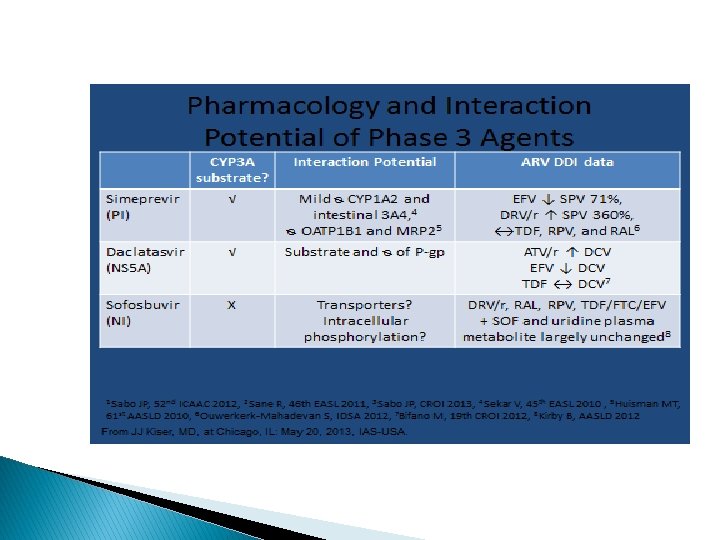
� HCV DAA (direct acting antiviral) Cheat Sheet ◦ PREVIR �Protease inhibitors: telaprevir, boceprevir, simeprevir ◦ BUVIR �Polymerase inhibitors �Sofosbuvir �ASvir �NS 5 A inhibitors: Daclatasvir, ledipasvir




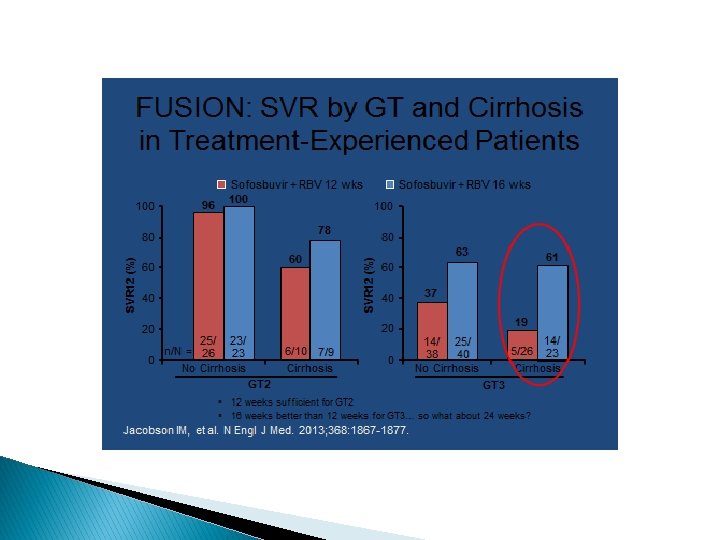
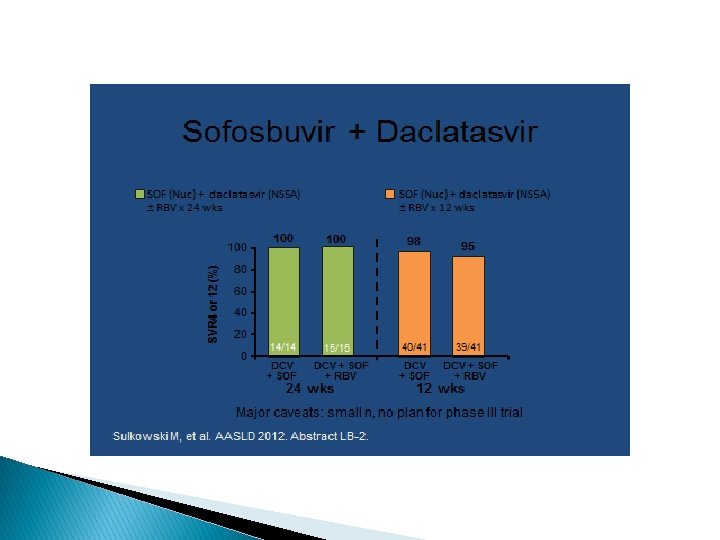
IDSA Recommendations � IFN-free: � Genotype 1: Sim/Sof x 12 weeks (+/- ribavirin) � Genotype 2: Sof/ribavirin x 12 weeks � Genotype 3: Sof/ribavirin x 24 weeks � Genotype 4: Sof/rifabirin x 24 weeks

Side effects: � Simeprevir ◦ Rash including photosensitivity (28%), itching (22%), nausea (22%), shortness of breath (12%), elevated bilirubin (49%) �Note rash more likely in patients with cirrhosis

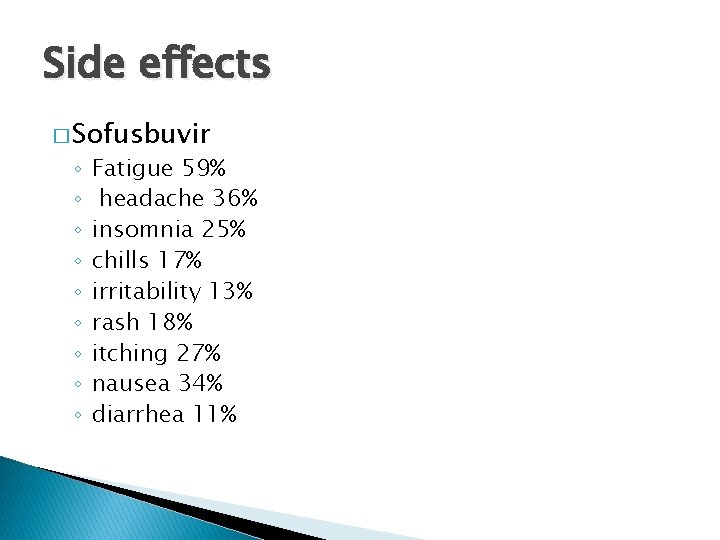
Side effects � Sofusbuvir ◦ ◦ ◦ ◦ ◦ Fatigue 59% headache 36% insomnia 25% chills 17% irritability 13% rash 18% itching 27% nausea 34% diarrhea 11%

Side effects of interferon


Side effects of simepravir/sofobuvir


� Overall <5% of study subjects stopped sofosbuvir and simeprevir on the COSMOS and other studies because of side effects.

Hep C meds to be approved � Daclatasvir: effective against genotypes 1, 2, 3 Ledipasvir: effective against genotype 1; to be combined with sofosbuvir into 1 pill a day



HIV/Hep C Drug interactions


� find those patients who need to be treated NOW with simeprevir/sofosbuvir, and who are willing to be on a limited HIV regimen (complera, isentress, truvada) in order to prevent drug interactions � Patients who are on Atripla, Stribild, or boosted protease inhibitors will have to wait until more hep C drugs available.

� Treat HIV first if CD 4 <500 and get HIV viral load <50 copies for maximal response from hep C meds � If CD 4 count >500, may be able to wait on starting HIV meds until after hep C treatment completed.

Treatment Schema

� Obtain baseline hep C viral load (within 3 months of beginning treatment) � Counsel patient on need to take all meds � Counsel patient on need to avoid sunlight, risks of nausea and rash � Alter patient’s HIV regimen if necessary.

� Follow-up 2 weeks and at 4 week point after initiating hep C meds to check on adherence and immediate side effects. Mild rash can be treated through with benedryl, topical steroids � Check CBC, platelets, AST, ALT every 2 weeks during first 4 weeks. � Repeat Hep C viral load at Week 4 point

� Hep C viral load should be <25 copies at Week 4 point; if not, patient may need to be discontinued to prevent resistance. � Recheck � If hep c viral load at Week 8. hep c meds tolerated, see patient at Week 4, Week 8 and Week 12 and check CBC, platelets, AST, and ALT at each visit(or monthly if being treated x 24 weeks)

� If patient on ribavirin containing regimen, dose reduce ribavirin if anemia develops: Hb < 10 � Most anemia with Sof/ribavirin mild.

� Repeat Hep C viral load 6 months after completing therapy to ascertain cure: “SVR”.

Audience Response Question: Which is FALSE 1)hep C viral load of 2, 000 copies may be a patient undergoing self-cure 2)a patient who is cured of hep C but still ha cirrhosis has no risk of liver cancer 3)simeprevir has multiple drug interactions with many HIV medications 4)patients cured of hep C have less fatigue

Reinfection

Study of reinfection rates � 191 MSM with cured (treated with meds) or spontaneously cleared hep C ◦ ◦ ◦ 44 were reinfected 8 were infected several more times Same or different genotypes None had IDU risk factor Estimated that 25% will be re-infected within 2 years of cure. ◦ AIDS 2013 Oct 23; 27(16): 2551 -7

� Prison populations in Spain: ◦ 119 Hep C Ab+, cured or spontaneously cleared while as inmate. 81% hx of IDU ◦ Reinfection rate 5. 37 per 100 person years, higher in active IDU and HIV co-infected ◦ J Hepatology. 2013 July; 59(1): 45 -51

� Selection of patients for hep C treatment will have to include safe sex counseling and sobriety

Conclusions

� Interferon free hep C drugs are here, and more coming � Be prepared for elaborate PA process to get the meds � Treatment will reduce complications of hep C infection and improve quality of life � Select patients who are not likely to get reinfected and will adhere to frequent clinic visits during treatment.

 Ardis moe
Ardis moe 学乐网moe
学乐网moe ........ is an alternative of log based recovery.
........ is an alternative of log based recovery. Hcv treatment
Hcv treatment Hcv window period
Hcv window period Hcv treatment
Hcv treatment Hcv symptoms female
Hcv symptoms female Manufacture of metallurgical coke by otto hoffman method
Manufacture of metallurgical coke by otto hoffman method Hcv
Hcv Ardis login
Ardis login Mark ardis
Mark ardis Child care moe
Child care moe Primary secondary and tertiary care
Primary secondary and tertiary care Davy crockett and the frozen dawn
Davy crockett and the frozen dawn Anne hathaway duffy
Anne hathaway duffy Kiko moe
Kiko moe Subjek nilam
Subjek nilam Nash moe
Nash moe Moe lim unc
Moe lim unc Sls moe
Sls moe Moe edusave character award
Moe edusave character award Course induction ntu
Course induction ntu Abraham wald
Abraham wald Moe suleiman
Moe suleiman Moe application window
Moe application window Professional practice guidelines moe
Professional practice guidelines moe Sistem i-nilam
Sistem i-nilam Moe archived dmca
Moe archived dmca Nowadays people are mỏe
Nowadays people are mỏe Moe character award
Moe character award Jpp moe
Jpp moe Moe nin alt unsuru olan el kitabı
Moe nin alt unsuru olan el kitabı Moe cockpit plus
Moe cockpit plus Sangamam moe
Sangamam moe Moe deep learning
Moe deep learning Almanhal moe ae
Almanhal moe ae Aed t&l
Aed t&l Ppdkerian
Ppdkerian Dziecko kochane
Dziecko kochane Xuele moe sg
Xuele moe sg Apdm pemulihan khas
Apdm pemulihan khas Standard 3 care certificate
Standard 3 care certificate Health and social care unit 2
Health and social care unit 2 Care sunt simturile prin care sunt evocate
Care sunt simturile prin care sunt evocate Polii magnetici de acelasi nume se
Polii magnetici de acelasi nume se Palliative care versus hospice care
Palliative care versus hospice care Care certificate answers standard 10
Care certificate answers standard 10 Inmultirea animalelor
Inmultirea animalelor Hip fracture care clinical care standard
Hip fracture care clinical care standard Webirb
Webirb Scala ucla npi
Scala ucla npi Ron hays ucla
Ron hays ucla Ucla applicant portal
Ucla applicant portal Dr gelabert ucla
Dr gelabert ucla