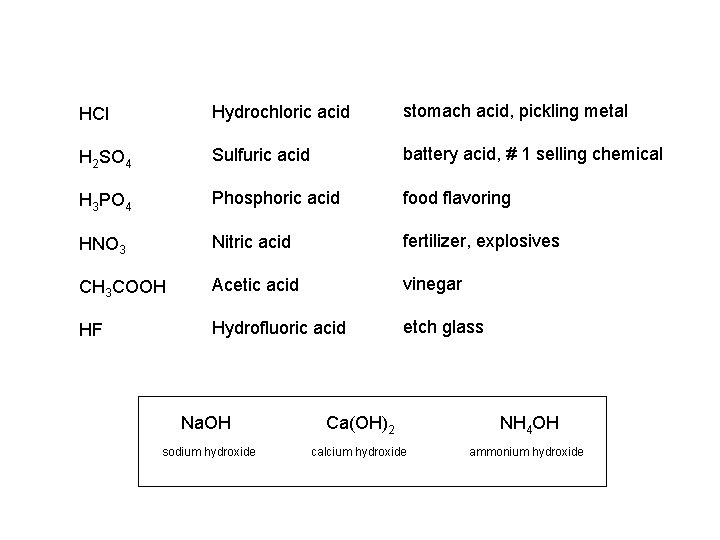
HCl Hydrochloric acid stomach acid pickling metal H
![Sorenson developed p. H scale 7 neutral p. H scale 0 [H+] = [OH-] Sorenson developed p. H scale 7 neutral p. H scale 0 [H+] = [OH-]](https://slidetodoc.com/presentation_image_h/e463d16098d12ea3ebfeae4891bed254/image-2.jpg)



![p. H Scale p. H = -log[H 3 p. OH = + O] -log[OH p. H Scale p. H = -log[H 3 p. OH = + O] -log[OH](https://slidetodoc.com/presentation_image_h/e463d16098d12ea3ebfeae4891bed254/image-25.jpg)

- Slides: 28

HCl Hydrochloric acid stomach acid, pickling metal H 2 SO 4 Sulfuric acid battery acid, # 1 selling chemical H 3 PO 4 Phosphoric acid food flavoring HNO 3 Nitric acid fertilizer, explosives CH 3 COOH Acetic acid vinegar HF Hydrofluoric acid etch glass Na. OH Ca(OH)2 NH 4 OH sodium hydroxide calcium hydroxide ammonium hydroxide
![Sorenson developed p H scale 7 neutral p H scale 0 H OH Sorenson developed p. H scale 7 neutral p. H scale 0 [H+] = [OH-]](https://slidetodoc.com/presentation_image_h/e463d16098d12ea3ebfeae4891bed254/image-2.jpg)
Sorenson developed p. H scale 7 neutral p. H scale 0 [H+] = [OH-] acid 14 base (alkalinity) Arnold Beckman invented the p. H meter p. H = -log [H+] p. OH = -log [OH-] p. H + p. OH = 14 k. W = [H+] [OH-] kw = 1 x 10 -14 H+ + H 2 O proton H 3 O+ hydronium ion

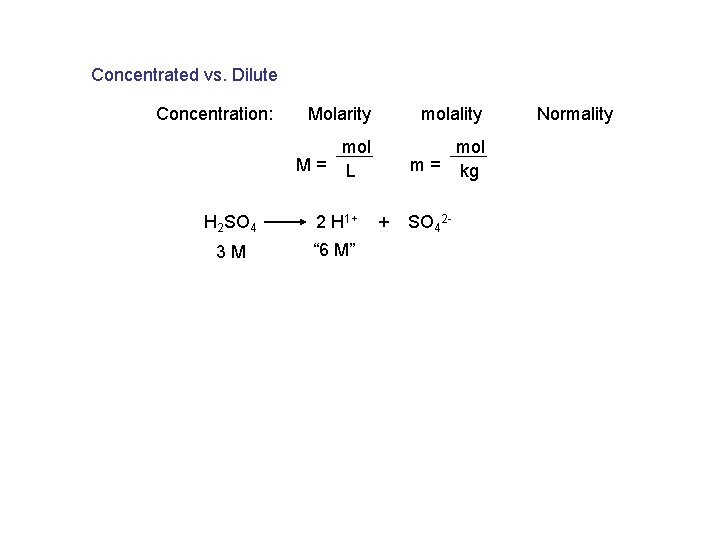
Concentrated vs. Dilute Concentration: Molarity molality mol M= L mol m = kg H 2 SO 4 2 H 1+ 3 M “ 6 M” + SO 42 - Normality

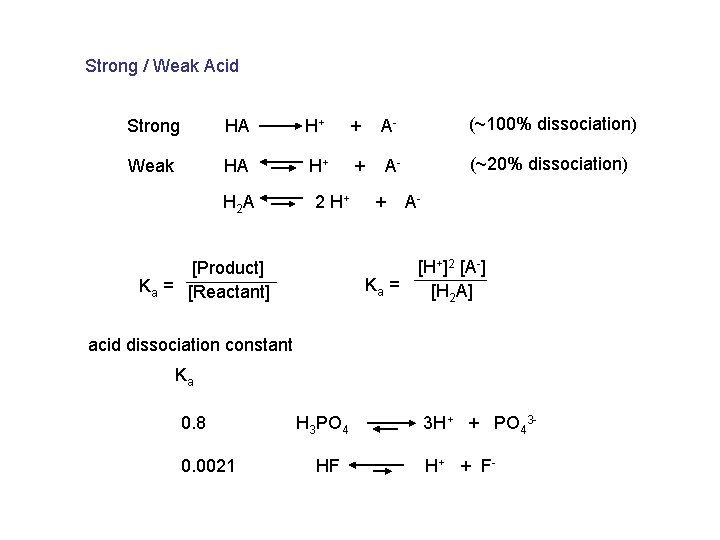
Strong / Weak Acid Strong HA H+ + A- (~100% dissociation) Weak HA H+ + A- (~20% dissociation) H 2 A 2 H+ [Product] Ka = [Reactant] + Ka = A[H+]2 [A-] [H 2 A] acid dissociation constant Ka 0. 8 0. 0021 H 3 PO 4 HF 3 H+ + PO 43 H + + F-

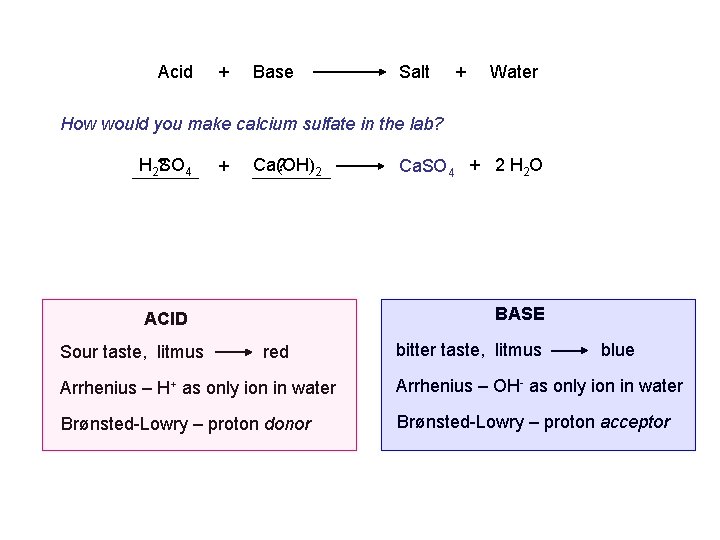
Acid + Base Salt + Water How would you make calcium sulfate in the lab? H 2? SO 4 + Ca(OH) ? 2 BASE ACID Sour taste, litmus Ca. SO 4 + 2 H 2 O red bitter taste, litmus blue Arrhenius – H+ as only ion in water Arrhenius – OH- as only ion in water Brønsted-Lowry – proton donor Brønsted-Lowry – proton acceptor

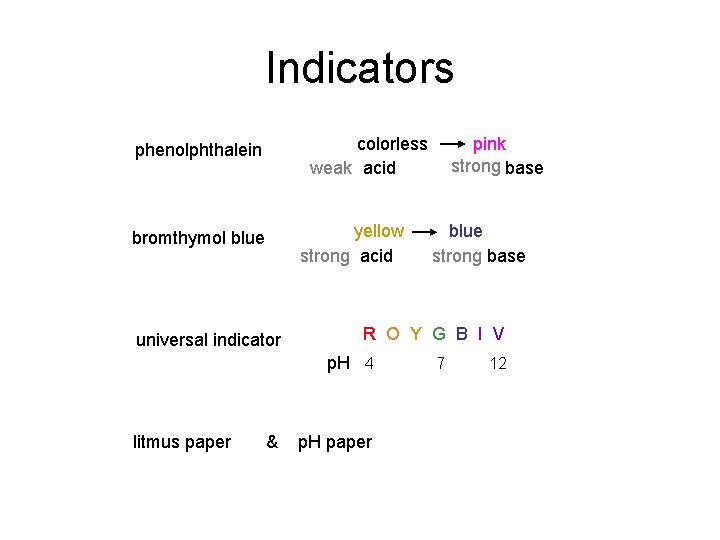
Indicators colorless weak acid phenolphthalein yellow strong acid bromthymol blue universal indicator & blue strong base R O Y G B I V p. H 4 litmus paper pink strong base p. H paper 7 12

Buffers - salts of weak acids and weak bases that maintain a p. H e. g. Aspirin (acetyl salicylic acid) vs. Bufferin low p. H upsets stomach Le. Chatelier’s Principle - acidosis & alkalosis (bicarbonate ion acts as buffer) - darkening glasses - egg shells thinner in summer (warm)

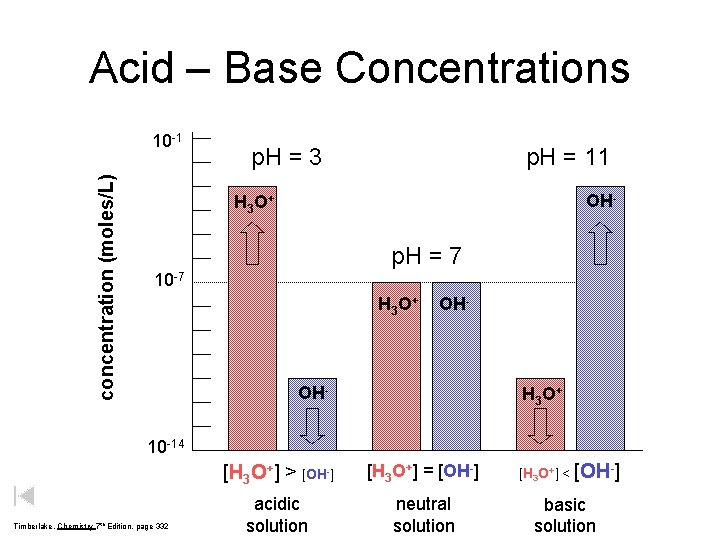
Acid – Base Concentrations concentration (moles/L) 10 -1 p. H = 3 p. H = 11 OH- H 3 O + p. H = 7 10 -7 H 3 O + OH- H 3 O + 10 -14 Timberlake, Chemistry 7 th Edition, page 332 [H 3 O+] > [OH-] [H 3 O+] = [OH-] acidic solution neutral solution [H 3 O+] < [OH-] basic solution

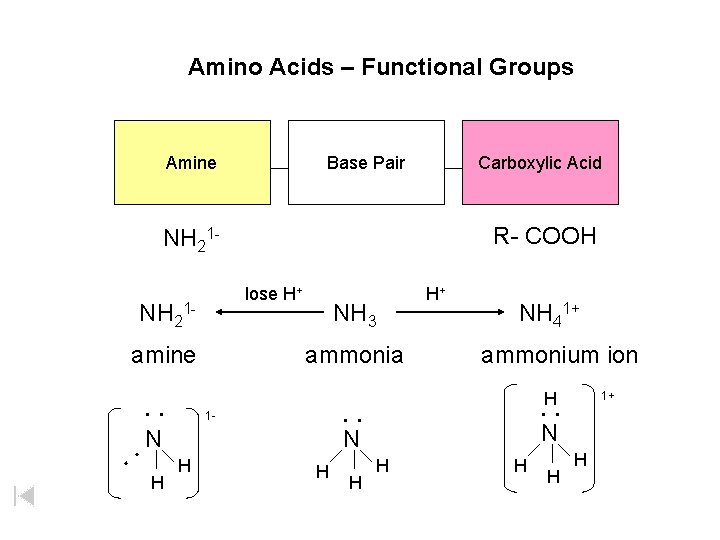
Amino Acids – Functional Groups Amine Base Pair Carboxylic Acid R- COOH NH 21 lose H+ NH 21 amine NH 3 ammonia H+ NH 41+ ammonium ion H : N H : : : 1 - N N H H H 1+ H H

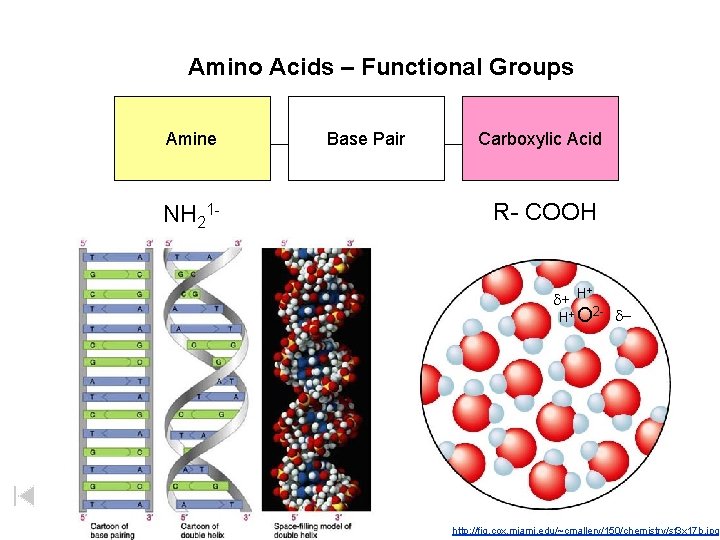
Amino Acids – Functional Groups Amine NH 21 - Base Pair Carboxylic Acid R- COOH + d+ H H+ O 2 - d- http: //fig. cox. miami. edu/~cmallery/150/chemistry/sf 3 x 17 b. jpg

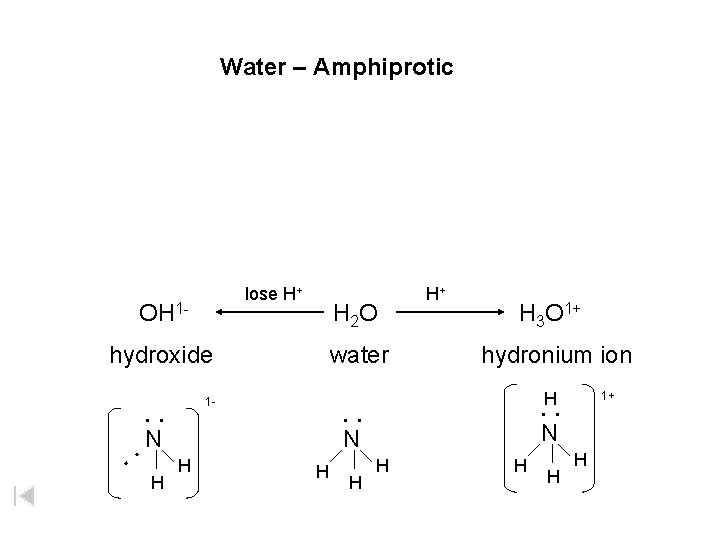
Water – Amphiprotic lose H+ OH 1 - H 2 O hydroxide water H+ H 3 O 1+ hydronium ion H N H H 1+ : : N : : 1 - N H H

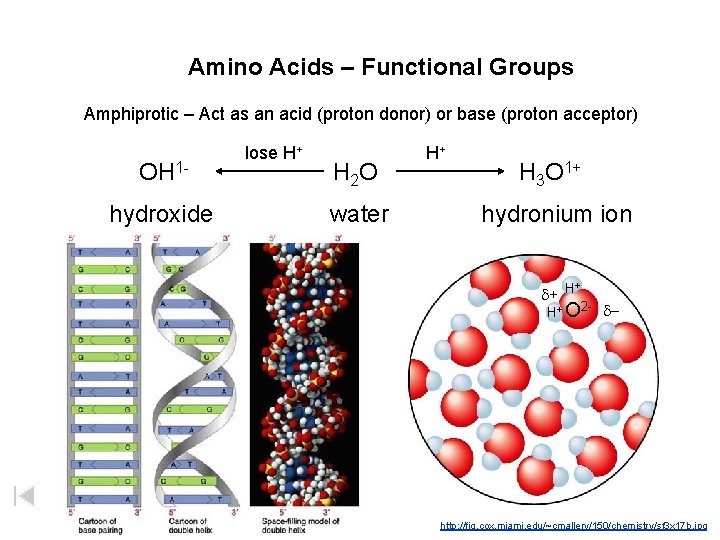
Water – Also Amphiprotic – Act as an acid (proton donor) or base (proton acceptor) OH 1 hydroxide lose H+ H 2 O water H+ H 3 O 1+ hydronium ion + d+ H H+ O 2 - d-

Amino Acids – Functional Groups Amphiprotic – Act as an acid (proton donor) or base (proton acceptor) OH 1 hydroxide lose H+ H 2 O water H+ H 3 O 1+ hydronium ion + d+ H H+ O 2 - d- http: //fig. cox. miami. edu/~cmallery/150/chemistry/sf 3 x 17 b. jpg

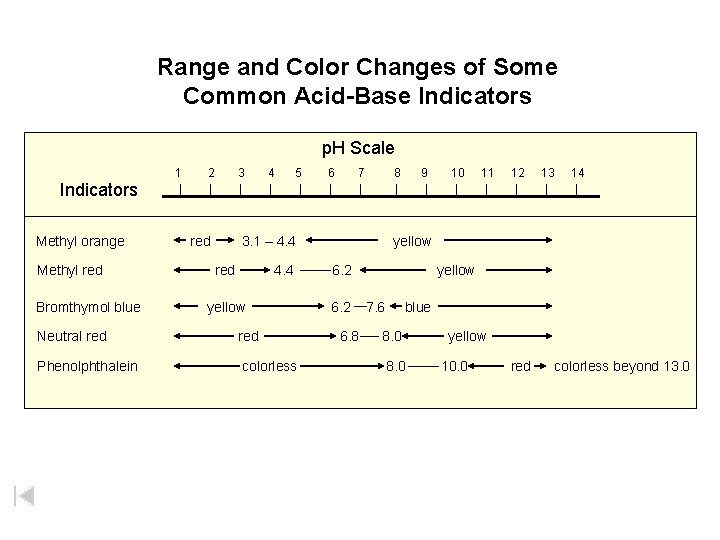
Range and Color Changes of Some Common Acid-Base Indicators p. H Scale 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Indicators Methyl orange Methyl red Bromthymol blue red 3. 1 – 4. 4 red 4. 4 yellow Neutral red Phenolphthalein colorless yellow 6. 2 6. 8 yellow 7. 6 blue 8. 0 yellow 10. 0 red colorless beyond 13. 0

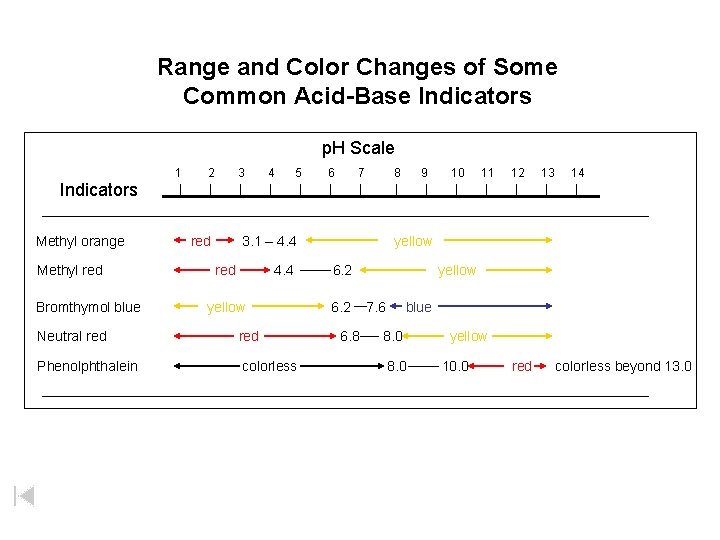
Range and Color Changes of Some Common Acid-Base Indicators p. H Scale 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Indicators Methyl orange Methyl red Bromthymol blue red 3. 1 – 4. 4 red 4. 4 yellow Neutral red Phenolphthalein colorless yellow 6. 2 6. 8 yellow 7. 6 blue 8. 0 yellow 10. 0 red colorless beyond 13. 0

p. H Paper p. H 0 1 2 3 4 5 p. H 7 8 9 10 11 12 6 p. H 0 1 2 3 4 5 13 p. H 7 8 9 10 11 12 13 6 p. H Paper p. H 0 1 2 3 4 5 p. H 7 8 9 10 11 12 6 p. H 0 1 2 3 4 5 13 p. H 7 8 9 10 11 12 13

Neutralization of Bug Bites Wasp - stings with base Red Ant - bites with acid (neutralize with lemon juice or vinegar) (neutralize with baking soda)

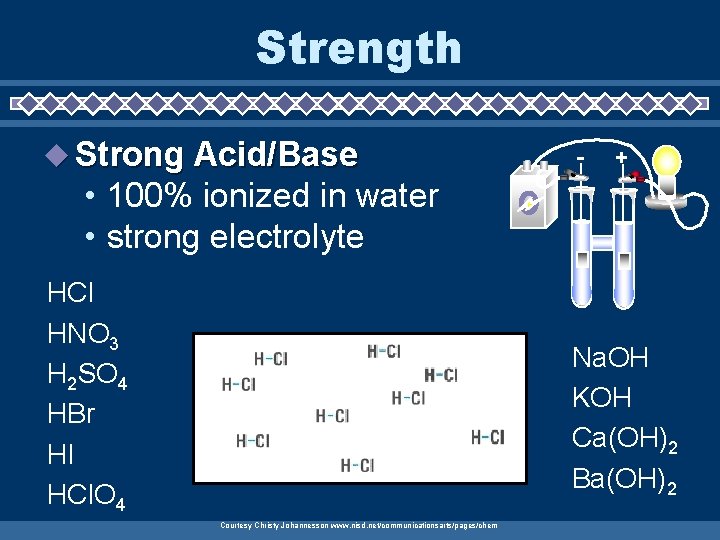
Strength u Strong Acid/Base - + • 100% ionized in water • strong electrolyte HCl HNO 3 H 2 SO 4 HBr HI HCl. O 4 Na. OH KOH Ca(OH)2 Ba(OH)2 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

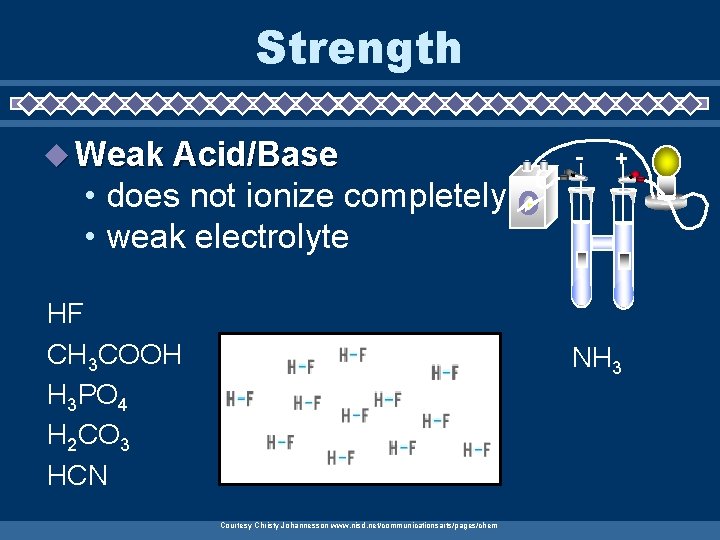
Strength u Weak Acid/Base - + • does not ionize completely • weak electrolyte HF CH 3 COOH H 3 PO 4 H 2 CO 3 HCN NH 3 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

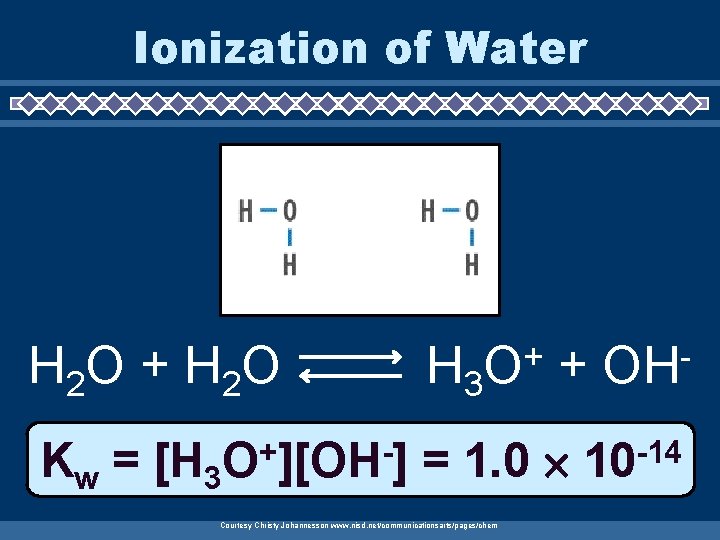
Ionization of Water H 2 O + H 2 O Kw = [H 3 + O ][OH ] H 3 + O + = 1. 0 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem OH -14 10

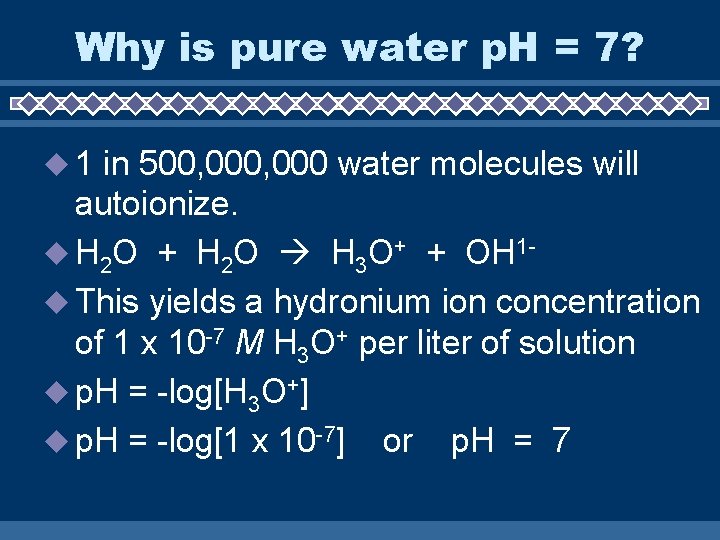
Why is pure water p. H = 7? u 1 in 500, 000 water molecules will autoionize. u H 2 O + H 2 O H 3 O+ + OH 1 u This yields a hydronium ion concentration of 1 x 10 -7 M H 3 O+ per liter of solution u p. H = -log[H 3 O+] u p. H = -log[1 x 10 -7] or p. H = 7

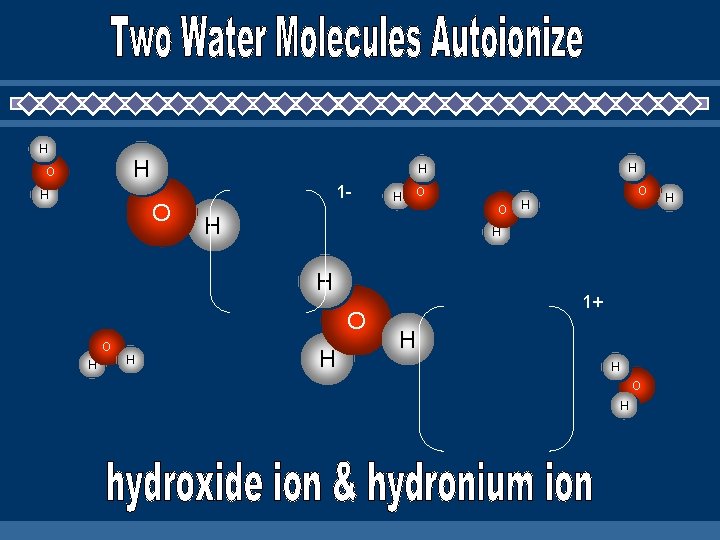
H H O 1 - H H O O O H H H 1+ H H O H H

Ionization of Water u Find the hydroxide ion concentration of 3. 0 10 -2 M HCl. [H 3 O+][OH-] = 1. 0 10 -14 [3. 0 10 -2][OH-] = 1. 0 10 -14 [OH-] = 3. 3 10 -13 M Acidic or basic? Acidic Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

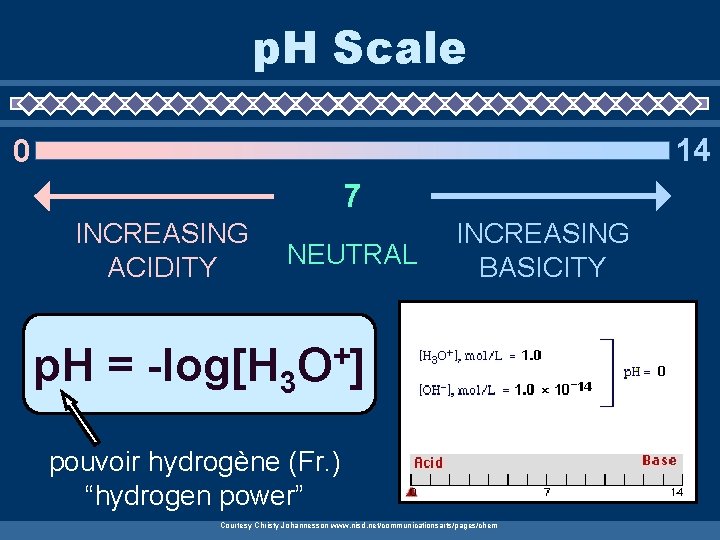
p. H Scale 14 0 7 INCREASING ACIDITY NEUTRAL p. H = -log[H 3 INCREASING BASICITY + O] pouvoir hydrogène (Fr. ) “hydrogen power” Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
![p H Scale p H logH 3 p OH O logOH p. H Scale p. H = -log[H 3 p. OH = + O] -log[OH](https://slidetodoc.com/presentation_image_h/e463d16098d12ea3ebfeae4891bed254/image-25.jpg)
p. H Scale p. H = -log[H 3 p. OH = + O] -log[OH ] p. H + p. OH = 14 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

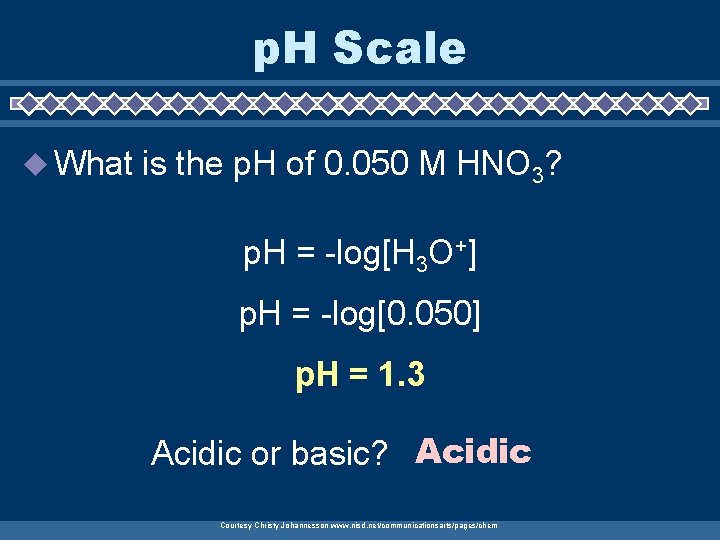
p. H Scale u What is the p. H of 0. 050 M HNO 3? p. H = -log[H 3 O+] p. H = -log[0. 050] p. H = 1. 3 Acidic or basic? Acidic Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

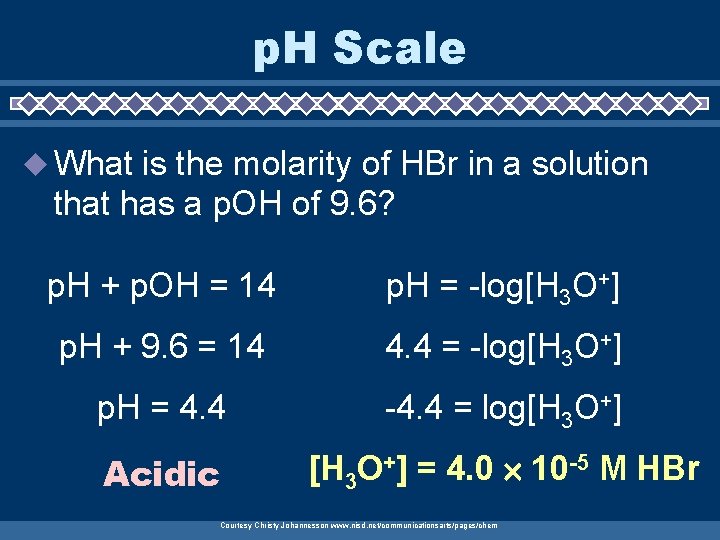
p. H Scale u What is the molarity of HBr in a solution that has a p. OH of 9. 6? p. H + p. OH = 14 p. H = -log[H 3 O+] p. H + 9. 6 = 14 4. 4 = -log[H 3 O+] p. H = 4. 4 -4. 4 = log[H 3 O+] Acidic [H 3 O+] = 4. 0 10 -5 M HBr Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

Resources - Acids and Bases Objectives Episode 16 – The Proton in Chemistry Worksheet - vocabulary Video (VHS) - future of the past Worksheet - p. H and p. OH calculations Outline - Worksheet - practice problems (key) Worksheet - Textbook - text ? 's chemical equilibrium Worksheet - weak acid, p. Ka Worksheet - Article - aspirin Worksheet - Lab - synthesis of aspirin Worksheet - aqueous acids and bases titration Lab - titration Textbook - questions Outline (general)