Hazardous Materials Incidents by Chris Hawley CHAPTER 6

Hazardous Materials Incidents by Chris Hawley CHAPTER 6: Product Control and Air Monitoring

Chapter 6: Overview • • • Introduction Defensive operations Air monitoring for first responders Meter methodology Oxygen monitors Combustible gas indicators Toxic gas monitors Other detectors Carbon monoxide incidents Summary 2

Defensive Operations • First responders should only use defensive methods to control releases of hazardous materials. – Entering the hot zone to take “hands on” action is not allowed. – You must have specific training to do other work. 3
Defensive Activities • • • Absorption Diking Damming Diverting Retention • • Dilution Vapor dispersion Vapor suppression Use of remote shutoffs 4

Absorption • Material must be compatible. • There is a variety of absorbing media available. • Spilled material may still present risk (flammable). 5

Diking • Must be sufficient size to collect material. • Minimum of three should be built. – Start away from the spill and work toward the spill. • Large spills require mechanical equipment. 6

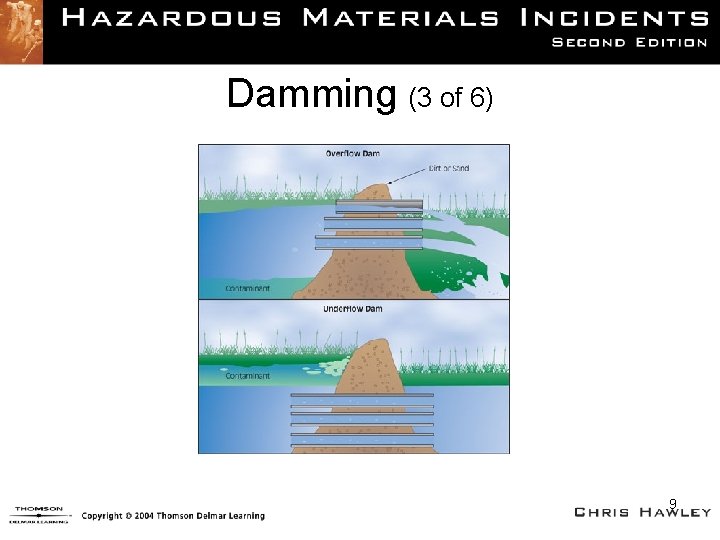
Damming (1 of 6) • There are two types. – Overflow – Underflow • At least three should be built. – Start away from spill and work toward spill. • Use mechanical equipment for large spills. 7

Damming (2 of 6) 8
Damming (3 of 6) 9

Damming (4 of 6) 10

Damming (5 of 6) 11

Damming (6 of 6) 12

Diverting • Commonly used to move a spill away from a risk area • Can use booms – Floating (absorbent) – Solid (harbor boom) 13

Retention • Retention is creating a hole to retain the spilled material. • Large spills require mechanical equipment. • Line hole with plastic or compatible material. 14

Dilution • Solution to pollution is not always dilution. – Non-soluble materials such as gasoline or fuels • Significant amounts of water are needed to neutralize corrosives. – May need several hundred thousand gallons for small spill 15

Vapor Dispersion • Creates water runoff issues • May not eliminate vapors – Just moves them • Requires large quantities of water 16

Vapor Suppression • Foam usually used – Must be reapplied on a regular basis – Must be compatible with the material 17

Remote Shutoffs • Tank trucks have emergency shutoffs. • Most tank facilities have remote shutoffs. • Responders should not endanger themselves to shut off valves. 18

Air Monitoring for the First Responder • Monitoring is essential to protect responders. – Fire risk – Toxic risk – Corrosive risk – Radiation risk 19

Regulations • It is OSHA’s intention that the worker (responder) be protected against workplace hazards. • Air monitors are the only way to identify potential airborne hazards. 20

Typical Configurations • Monitors usually have 3 -5 functions. – LEL sensor – Oxygen sensor – Carbon monoxide – Hydrogen sulfide – Other toxic material 21

Basic Principles of Air Monitoring & Detection Devices • Air monitors – Primary protection – Can assist with determining the presence of risk materials – Help provide a possible identity of the material 22

Air Monitoring Strategies • Always use p. H, LEL, O 2, and PID as a minimum. • Expand the use of other monitors as more information is available. • The absence of readings does not mean that harmful materials are not present. – Biological threat agents 23
Determination of Risk • Corrosive – p. H paper • Oxygen – Oxygen sensor • Fire – LEL or flammable gas detector • Toxic – Photoionization detector (PID) • Radioactive – Rad pager and monitors 24
Meter Terminology • Accuracy – Ability to produce findings close to actual quantity of gas • Precision – Ability to reproduce the same results • Factors affecting precision – Sensor technology – Weather 25
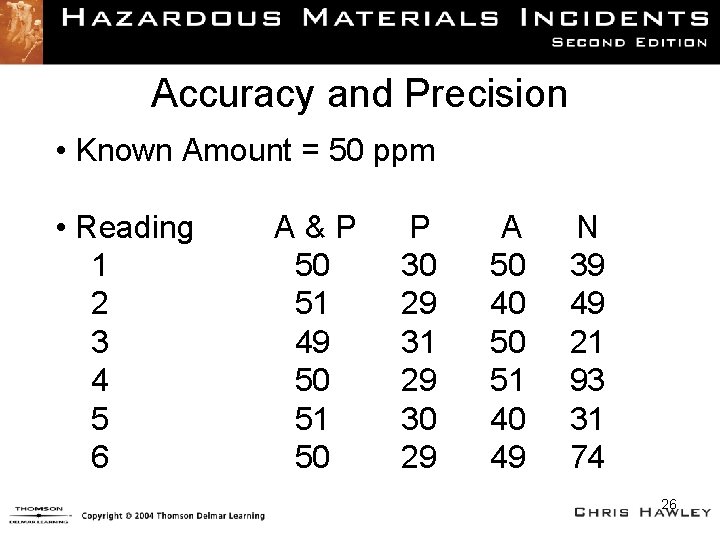
Accuracy and Precision • Known Amount = 50 ppm • Reading 1 2 3 4 5 6 A&P 50 51 49 50 51 50 P 30 29 31 29 30 29 A 50 40 50 51 40 49 N 39 49 21 93 31 74 26

Bump Test • Exposing monitor to known gases and allowing it to go into alarm • Also known as a field test • If insufficient response, conduct calibration 27
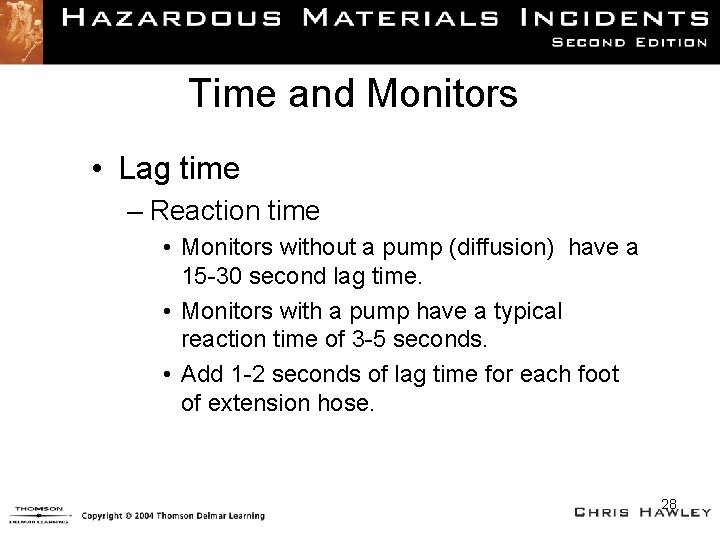
Time and Monitors • Lag time – Reaction time • Monitors without a pump (diffusion) have a 15 -30 second lag time. • Monitors with a pump have a typical reaction time of 3 -5 seconds. • Add 1 -2 seconds of lag time for each foot of extension hose. 28

Response Times • • • Photoionization = 1 -2 seconds LEL with pump = up to 7 seconds LEL w/o pump = up to 30 seconds CO and H 2 S sensors = >20 seconds Ion mobility = up to 1 ½ minutes Radiation monitors = up to 1 ½ minutes 29
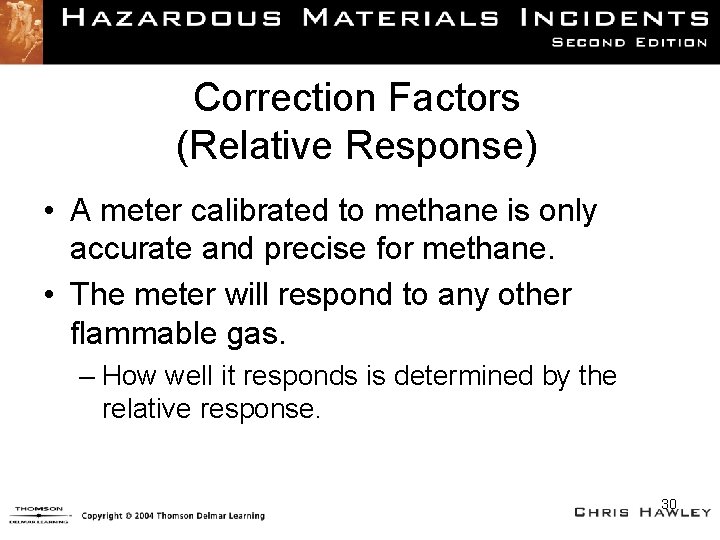
Correction Factors (Relative Response) • A meter calibrated to methane is only accurate and precise for methane. • The meter will respond to any other flammable gas. – How well it responds is determined by the relative response. 30
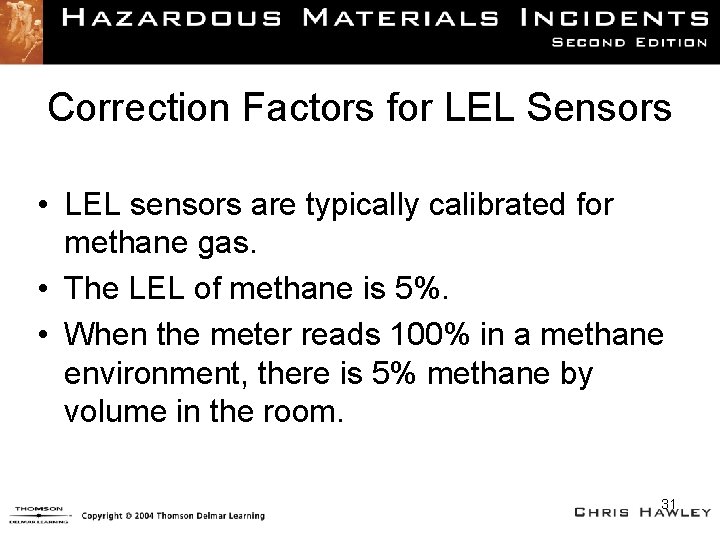
Correction Factors for LEL Sensors • LEL sensors are typically calibrated for methane gas. • The LEL of methane is 5%. • When the meter reads 100% in a methane environment, there is 5% methane by volume in the room. 31
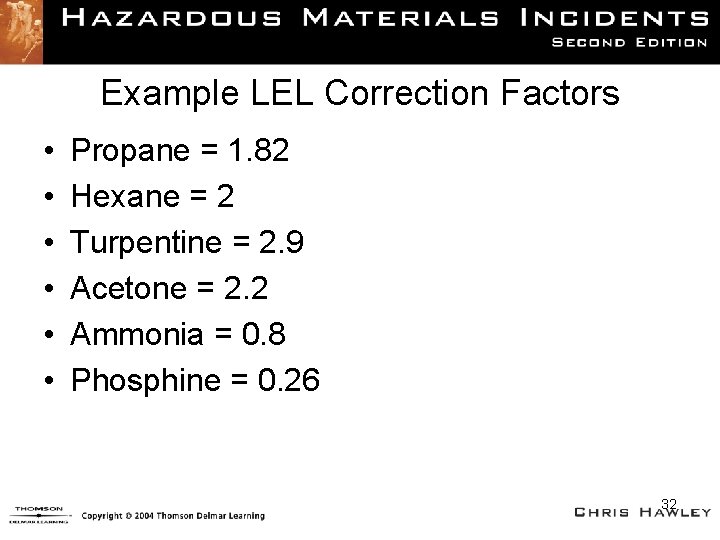
Example LEL Correction Factors • • • Propane = 1. 82 Hexane = 2 Turpentine = 2. 9 Acetone = 2. 2 Ammonia = 0. 8 Phosphine = 0. 26 32
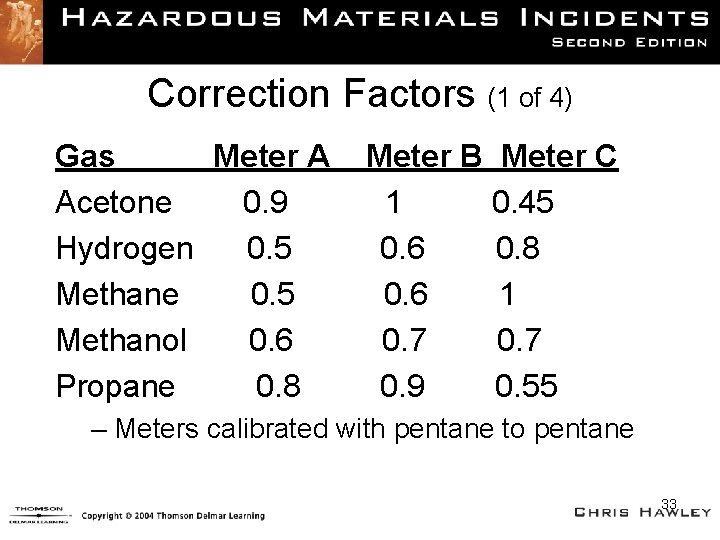
Correction Factors (1 of 4) Gas Meter A Acetone 0. 9 Hydrogen 0. 5 Methane 0. 5 Methanol 0. 6 Propane 0. 8 Meter B Meter C 1 0. 45 0. 6 0. 8 0. 6 1 0. 7 0. 9 0. 55 – Meters calibrated with pentane to pentane 33
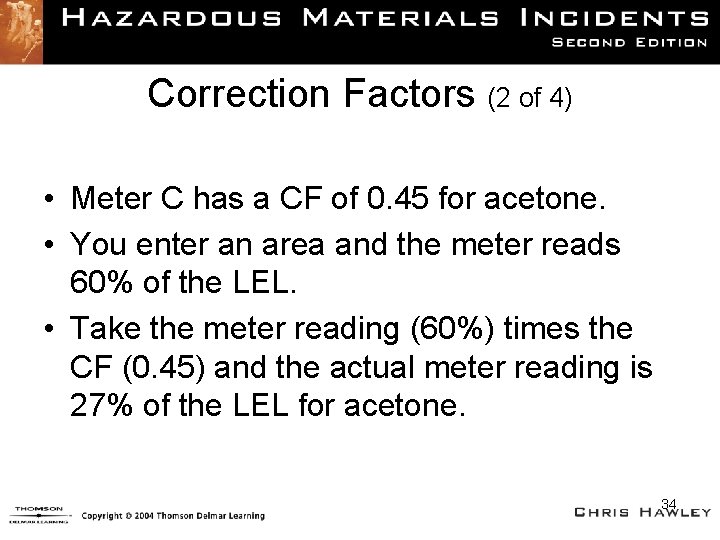
Correction Factors (2 of 4) • Meter C has a CF of 0. 45 for acetone. • You enter an area and the meter reads 60% of the LEL. • Take the meter reading (60%) times the CF (0. 45) and the actual meter reading is 27% of the LEL for acetone. 34
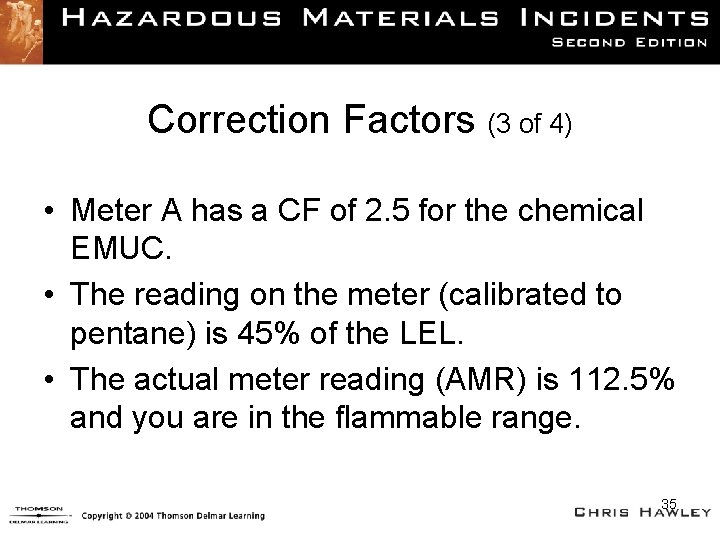
Correction Factors (3 of 4) • Meter A has a CF of 2. 5 for the chemical EMUC. • The reading on the meter (calibrated to pentane) is 45% of the LEL. • The actual meter reading (AMR) is 112. 5% and you are in the flammable range. 35
Correction Factors (4 of 4) • Acetone is being used in a process. • Correction factor for acetone is 2. 2. • In the building, the meter reads 5% of the LEL. – Meter is calibrated to methane. • Multiply 5% times 2. 2 = 11%. • The meter should be reading 11%. 36
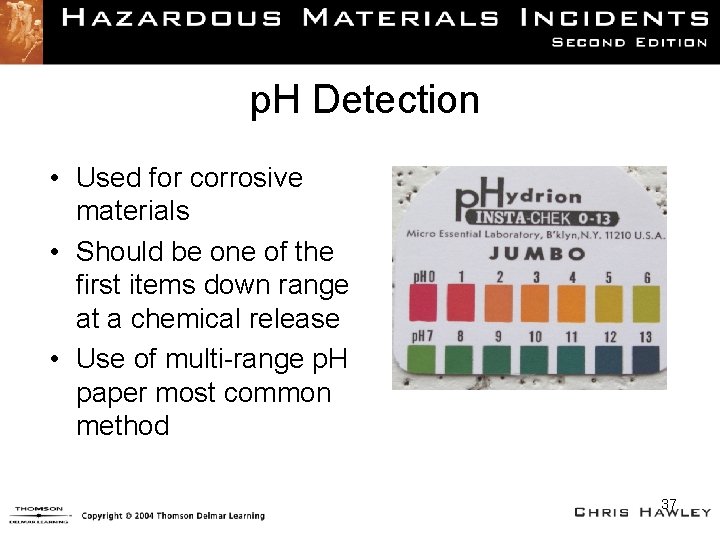
p. H Detection • Used for corrosive materials • Should be one of the first items down range at a chemical release • Use of multi-range p. H paper most common method 37
Oxygen Monitors • Normal air contains 20. 9% oxygen. • Less than 19. 5% oxygen is considered oxygen deficient. – LEL readings are off. • Greater than 23. 5% oxygen is oxygen enriched. – LEL readings are off. 38

Oxygen Sensor 39
Oxygen Sensor Limitations • Most oxygen sensors will only last 1 -2 years. • Chemicals with additional oxygen in their molecular structure hurt the sensors. • Optimal temperature is between 32° 120°F. 40
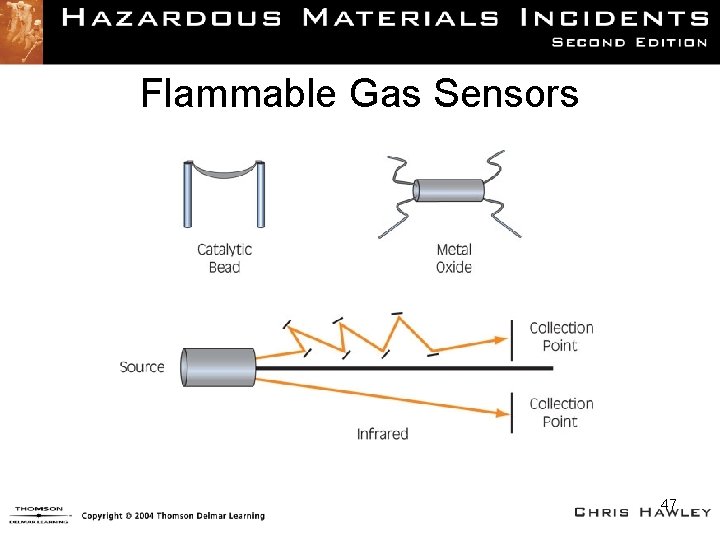
Flammable Gas Indicators (FGIs) • Also referred to as combustible gas sensors or LEL sensors • Used to measure the lower explosive limit (LEL) of the calibration gas • Majority calibrated to methane (natural gas), using pentane gas as the calibration standard – When calibrated for methane, the sensor will read up to the LEL of methane. 41
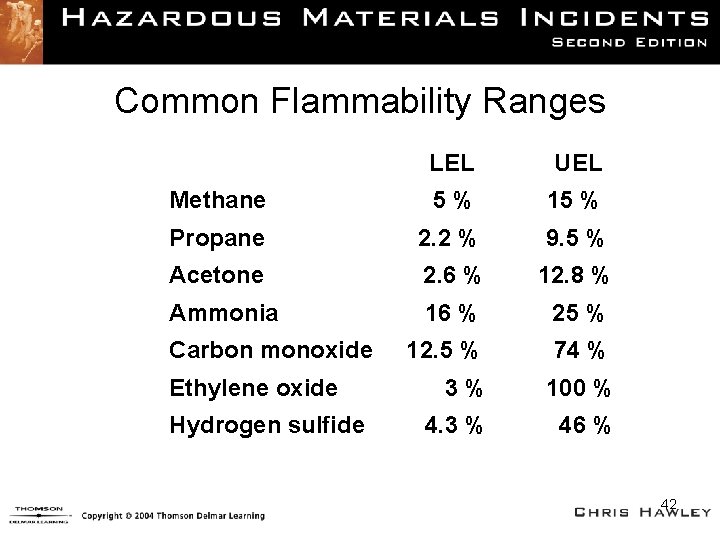
Common Flammability Ranges LEL UEL Methane 5% 15 % Propane 2. 2 % 9. 5 % Acetone 2. 6 % 12. 8 % Ammonia 16 % 25 % 12. 5 % 74 % 3% 100 % 4. 3 % 46 % Carbon monoxide Ethylene oxide Hydrogen sulfide 42

Catalytic (Pellistor) Bead Structure 43
LEL Sensor Types (1 of 2) • The basic principle of LEL sensors is that a stream of sampled air passes through the sensor housing causing a heat increase and conversely creating an electric charge, causing a reading on the instrument. 44
LEL Sensor Types (2 of 2) • Catalytic bead – Round piece of heated metal strung between a wire – Quick reaction time and precise sensor • Metal oxide sensor (MOS) – A semiconductor in a sealed unit that has a Wheatstone bridge in it surrounded by coating of a metal oxide 45
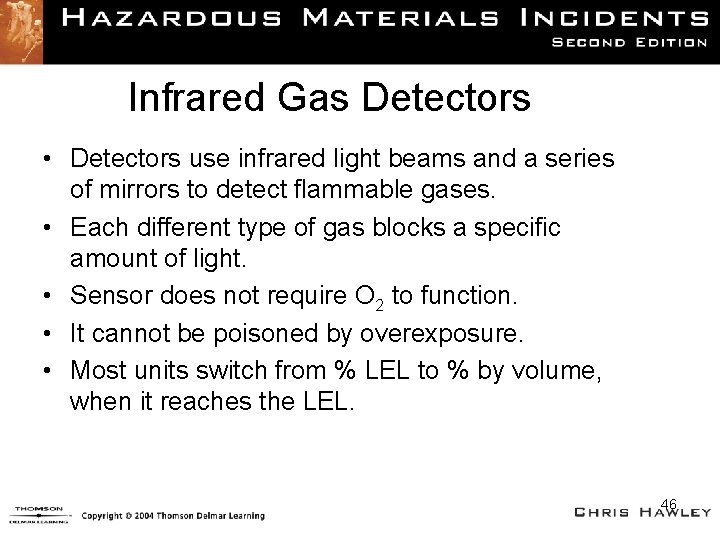
Infrared Gas Detectors • Detectors use infrared light beams and a series of mirrors to detect flammable gases. • Each different type of gas blocks a specific amount of light. • Sensor does not require O 2 to function. • It cannot be poisoned by overexposure. • Most units switch from % LEL to % by volume, when it reaches the LEL. 46
Flammable Gas Sensors 47

Toxic Gas Monitors • Toxic sensors are available in a variety of gases. – – – – – Carbon monoxide Hydrogen sulfide Chlorine Ammonia Sulfur dioxide Hydrogen chloride Hydrogen cyanide Nitrogen dioxide Many others 48

Toxic Sensors • Most are electrochemical sensors with electrodes (two or more) and chemical mixture sealed in a sensor housing. • The gases pass over the sensor causing a chemical reaction within the sensor. • Electrical charge is created which causes a readout to be displayed. 49

Electrochemical Toxic Sensor 50
Electrochemical Sensor Cross Sensitivity 51

Photoionization Detectors (PID) • Can detect a wide variety of gases in small amounts • Will not indicate what materials are present • Can identify potential areas of concern and possible leaks or contamination • Sensitivity from 0. 1 - 2, 000 PPM – Part per billion unit available 52
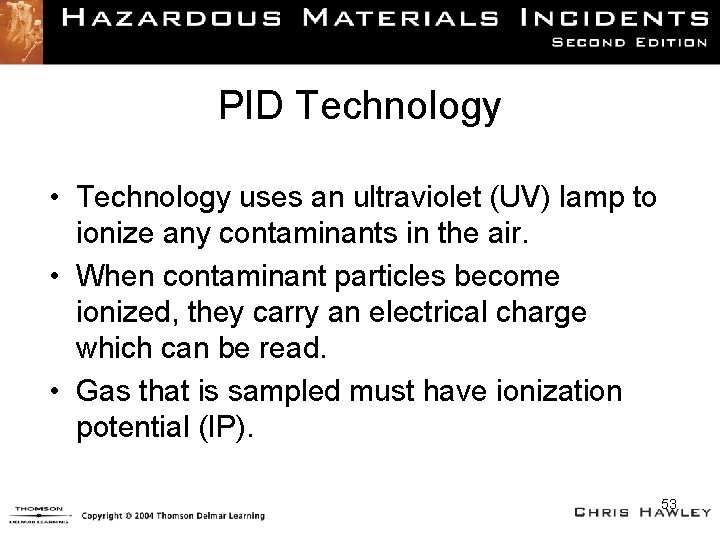
PID Technology • Technology uses an ultraviolet (UV) lamp to ionize any contaminants in the air. • When contaminant particles become ionized, they carry an electrical charge which can be read. • Gas that is sampled must have ionization potential (IP). 53
What Does a PID Measure? (1 of 2) 54
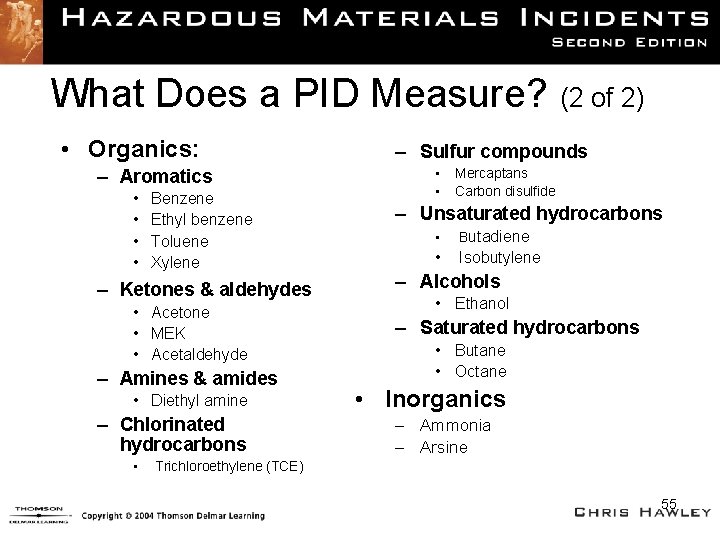
What Does a PID Measure? (2 of 2) • Organics: – Aromatics • • Benzene Ethyl benzene Toluene Xylene – Ketones & aldehydes • Acetone • MEK • Acetaldehyde – Amines & amides • Diethyl amine – Chlorinated hydrocarbons • – Sulfur compounds • • Mercaptans Carbon disulfide – Unsaturated hydrocarbons • Butadiene • Isobutylene – Alcohols • Ethanol – Saturated hydrocarbons • Butane • Octane • Inorganics – Ammonia – Arsine Trichloroethylene (TCE) 55
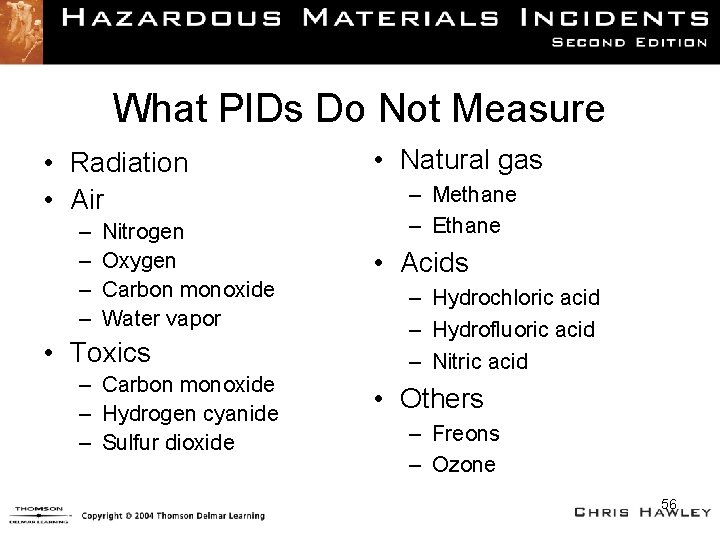
What PIDs Do Not Measure • Radiation • Air – – Nitrogen Oxygen Carbon monoxide Water vapor • Toxics – Carbon monoxide – Hydrogen cyanide – Sulfur dioxide • Natural gas – Methane – Ethane • Acids – Hydrochloric acid – Hydrofluoric acid – Nitric acid • Others – Freons – Ozone 56
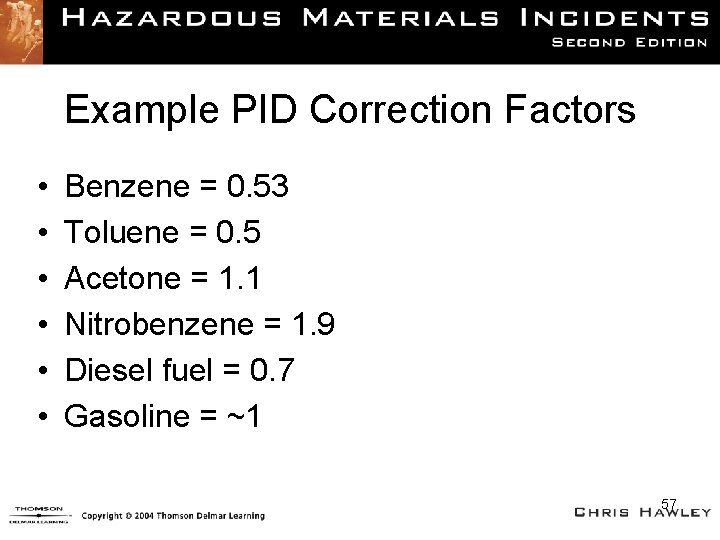
Example PID Correction Factors • • • Benzene = 0. 53 Toluene = 0. 5 Acetone = 1. 1 Nitrobenzene = 1. 9 Diesel fuel = 0. 7 Gasoline = ~1 57
CF Example: Toluene • Toluene CF with 10. 6 e. V lamp is 0. 5 – If PID calibrated to isobutylene reads 100 ppm in a Toluene atmosphere, then the actual concentration is 50 ppm Toluene units. • 0. 5 x 100 ppm= 50 ppm 58

Problems with PIDs • The lamps are affected by dirt and dust and require cleaning. • Higher levels of methane (natural gas, swamp gas, landfill gas) may suppress some readings. • Extreme humidity plays a role in the reading. 59
Colorimetric Sampling Types • Standard tubes • Multiple-test system • Chip measurement system 60
Standard Colorimetric Systems • Glass tubes filled with a reagent material change color when exposed to the intended gas. • Air sample is drawn across the tube in a specific quantity. • The tube changes color in the presence of the contaminant the tube is intended to detect. 61
Standard Tubes • Cover standard chemical families • Used to sample for identified and unidentified materials • Usually available in various sensitivities • Some bundled together in a manifold for simultaneous multiple sampling 62
Colorimetric Sampling • Chip system involves the use of barcoded sampling chips. – A sampling chip is inserted into a pump. • The pump recognizes the chip in use and provides the correct amount of sample through the reagent. – A reflective measurement provides an accurate reading of the gas that may be present. 63

Carbon Monoxide (CO) • Colorless, odorless, and tasteless • Only detectable through the use of air monitors – Your nose is not effective at all at detecting CO, no matter what odor may be present. 64

CO Symptoms • Exposure can present flu-like symptoms, headache, nausea, dizziness, confusion, and irritability. • Exposure to high levels can cause vomiting, chest pain, shortness of breath, loss of consciousness, brain damage, and death. • Amounts in the 9 ppm range have killed unborn babies in the womb. 65

Exposure Levels • OSHA provides that less than an average of 50 ppm for an 8 -hour period is acceptable. • NIOSH states 35 ppm for 10 hours. • ACGIH uses less than 25 ppm average for 8 hours. 66
Sources of CO • • • Furnaces (oil and gas) Hot water heaters (oil and gas) Fireplaces (wood, coal, and gas) Kerosene heaters (or other fueled heaters) Gasoline engines running inside (basements or garages) • Barbecue grills burning near the residence (garage or porch) • Faulty flues or exhaust pipes 67

Summary • • Defensive operations Air monitoring for first responders Meter methodology Oxygen monitors Combustible gas indicators Toxic gas monitors Other detectors Carbon monoxide incidents 68
- Slides: 68