Harmonic Oscillator Harmonic Oscillator Selections rules Permanent Dipole


- Slides: 21

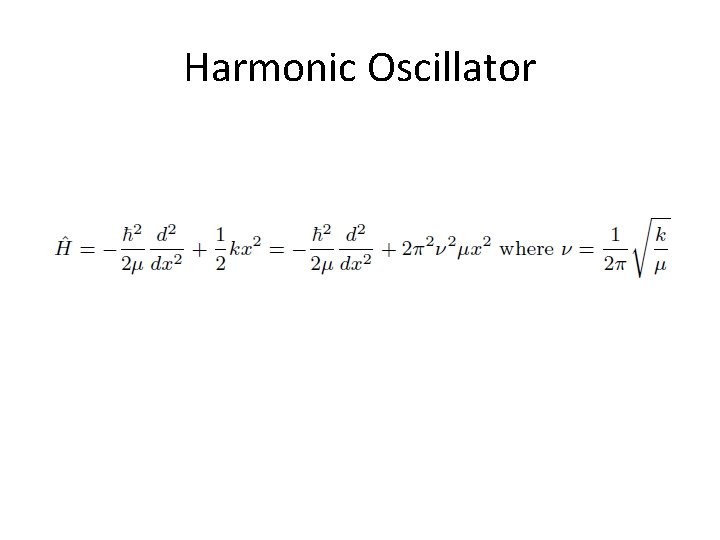
Harmonic Oscillator

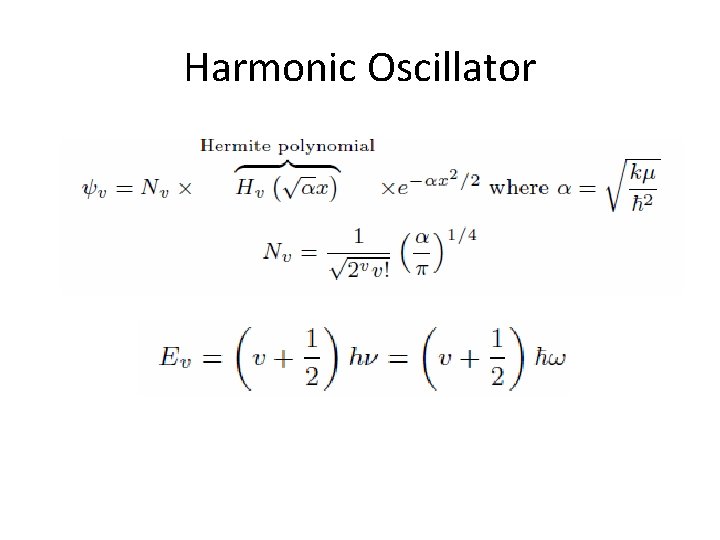
Harmonic Oscillator

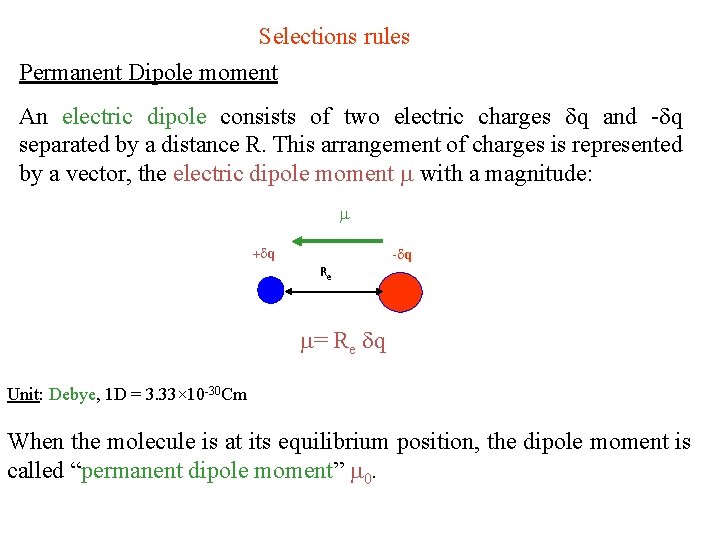
Selections rules Permanent Dipole moment An electric dipole consists of two electric charges q and - q separated by a distance R. This arrangement of charges is represented by a vector, the electric dipole moment with a magnitude: + q - q Re = Re q Unit: Debye, 1 D = 3. 33× 10 -30 Cm When the molecule is at its equilibrium position, the dipole moment is called “permanent dipole moment” 0.

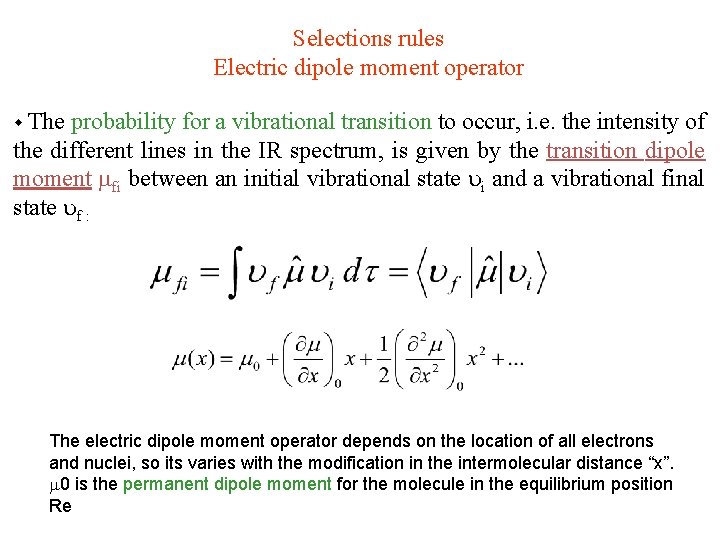
Selections rules Electric dipole moment operator The probability for a vibrational transition to occur, i. e. the intensity of the different lines in the IR spectrum, is given by the transition dipole moment fi between an initial vibrational state i and a vibrational final state f : The electric dipole moment operator depends on the location of all electrons and nuclei, so its varies with the modification in the intermolecular distance “x”. 0 is the permanent dipole moment for the molecule in the equilibrium position Re

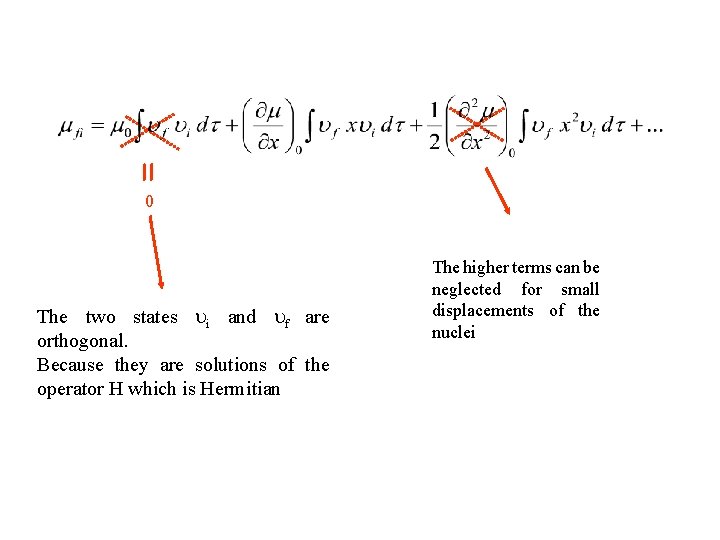
0 The two states i and f are orthogonal. Because they are solutions of the operator H which is Hermitian The higher terms can be neglected for small displacements of the nuclei

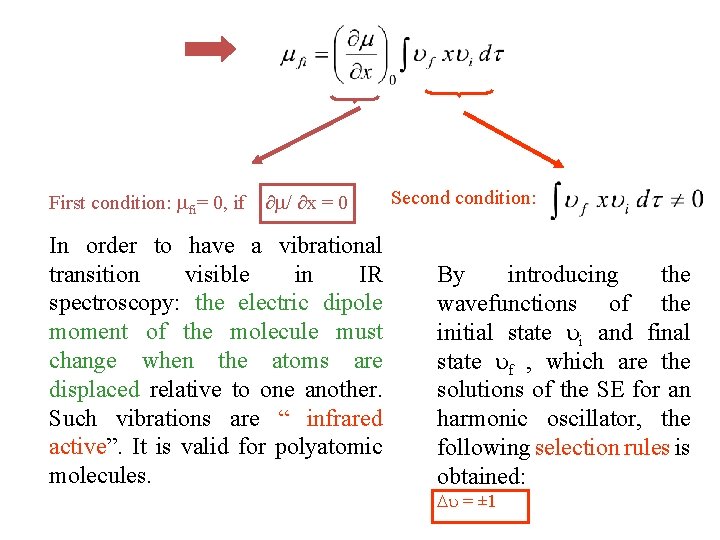
First condition: fi= 0, if ∂ / ∂x = 0 In order to have a vibrational transition visible in IR spectroscopy: the electric dipole moment of the molecule must change when the atoms are displaced relative to one another. Such vibrations are “ infrared active”. It is valid for polyatomic molecules. Secondition: By introducing the wavefunctions of the initial state i and final state f , which are the solutions of the SE for an harmonic oscillator, the following selection rules is obtained: = ± 1

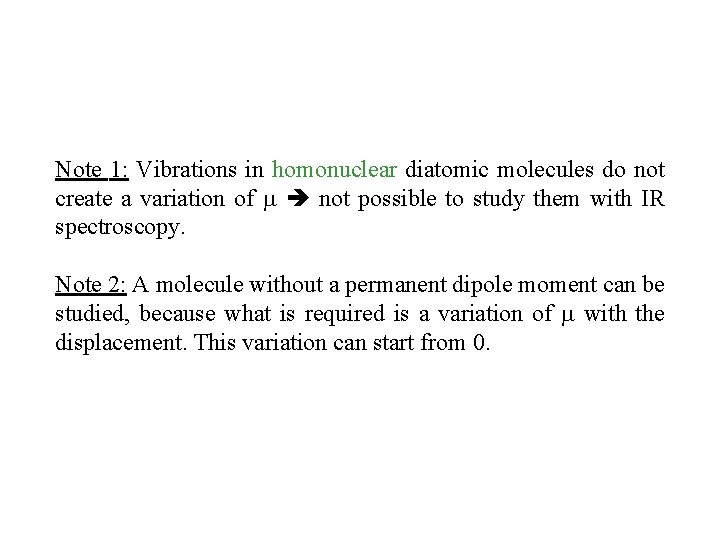
Note 1: Vibrations in homonuclear diatomic molecules do not create a variation of not possible to study them with IR spectroscopy. Note 2: A molecule without a permanent dipole moment can be studied, because what is required is a variation of with the displacement. This variation can start from 0.

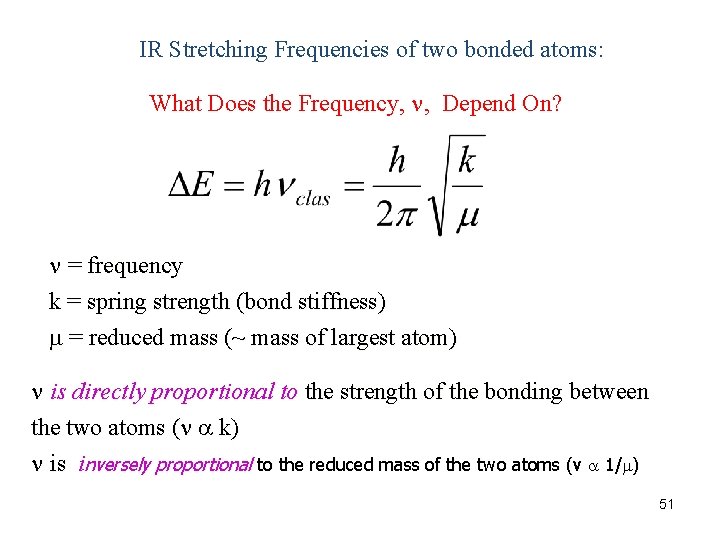
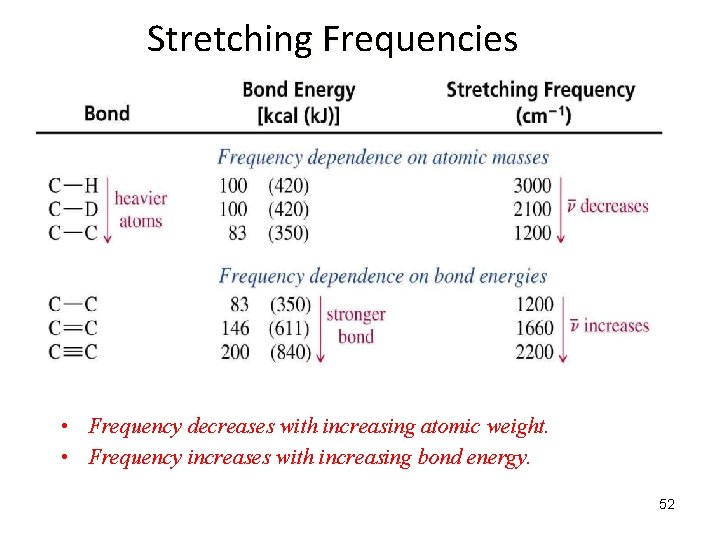
IR Stretching Frequencies of two bonded atoms: What Does the Frequency, , Depend On? = frequency k = spring strength (bond stiffness) = reduced mass (~ mass of largest atom) is directly proportional to the strength of the bonding between the two atoms ( k) is inversely proportional to the reduced mass of the two atoms (v 1/ ) 51

Stretching Frequencies • Frequency decreases with increasing atomic weight. • Frequency increases with increasing bond energy. 52

IR spectroscopy is an important tool in structural determination of unknown compound

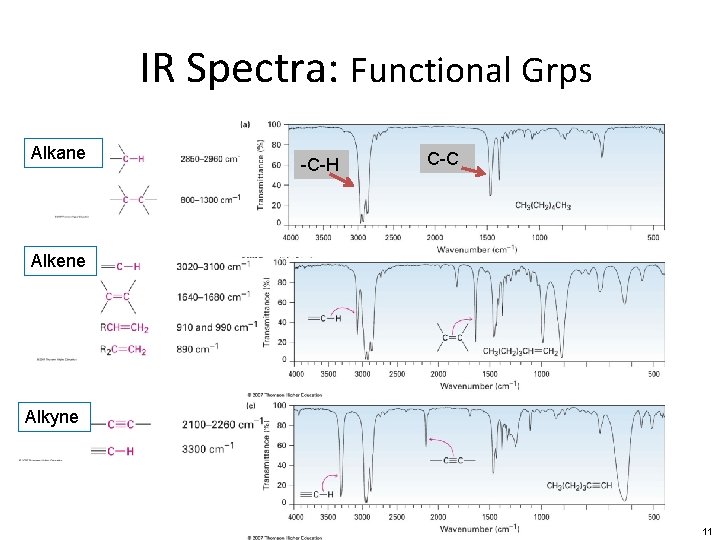
IR Spectra: Functional Grps Alkane -C-H C-C Alkene Alkyne 11

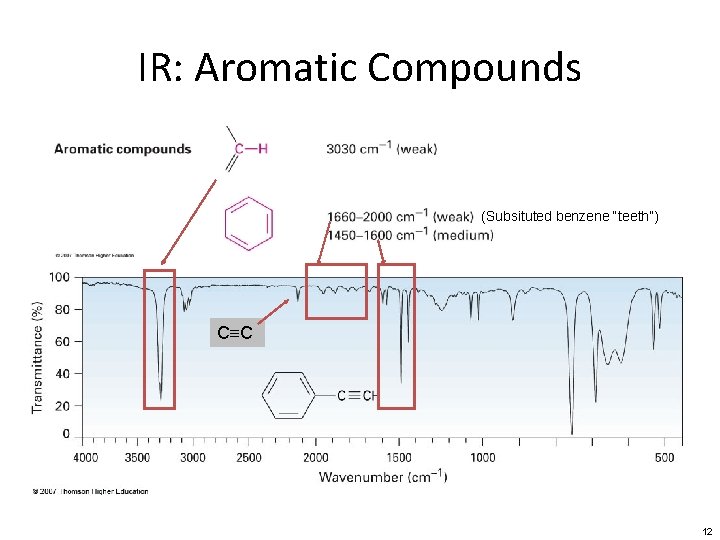
IR: Aromatic Compounds (Subsituted benzene “teeth”) C≡C 12

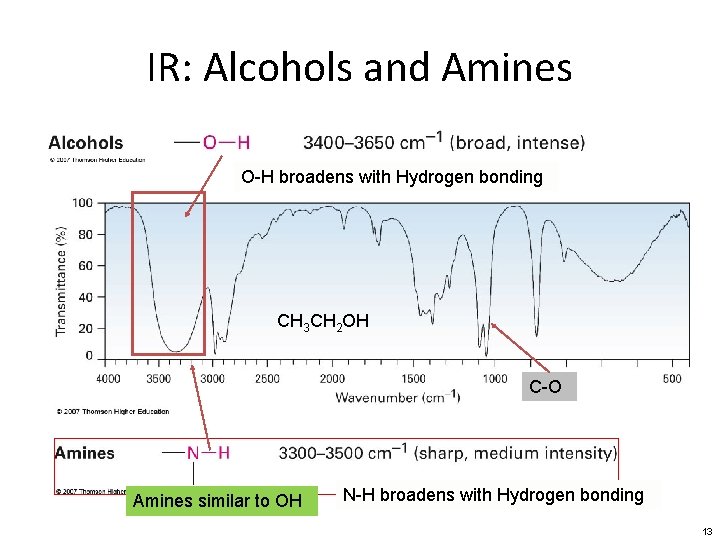
IR: Alcohols and Amines O-H broadens with Hydrogen bonding CH 3 CH 2 OH C-O Amines similar to OH N-H broadens with Hydrogen bonding 13

CO 2, A greenhouse gas ?

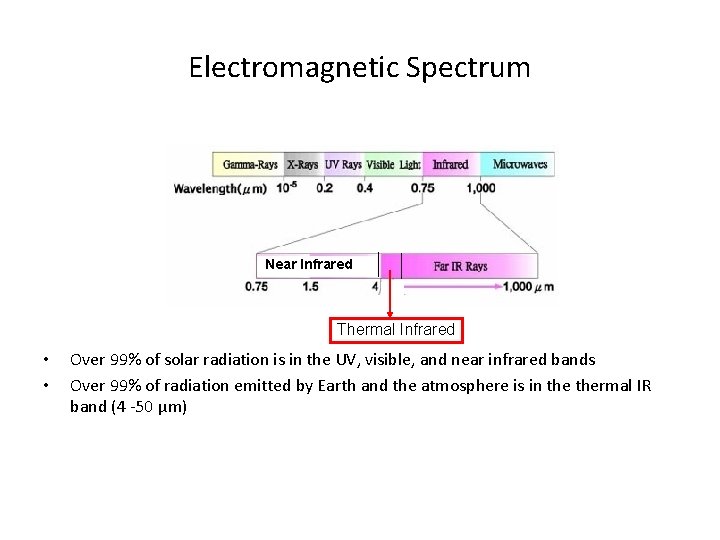
Electromagnetic Spectrum Near Infrared Thermal Infrared • • Over 99% of solar radiation is in the UV, visible, and near infrared bands Over 99% of radiation emitted by Earth and the atmosphere is in thermal IR band (4 -50 µm)

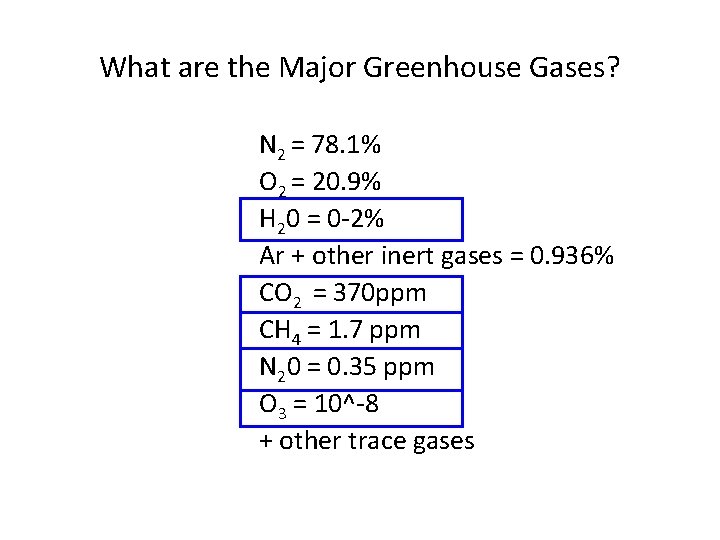
What are the Major Greenhouse Gases? N 2 = 78. 1% O 2 = 20. 9% H 20 = 0 -2% Ar + other inert gases = 0. 936% CO 2 = 370 ppm CH 4 = 1. 7 ppm N 20 = 0. 35 ppm O 3 = 10^-8 + other trace gases

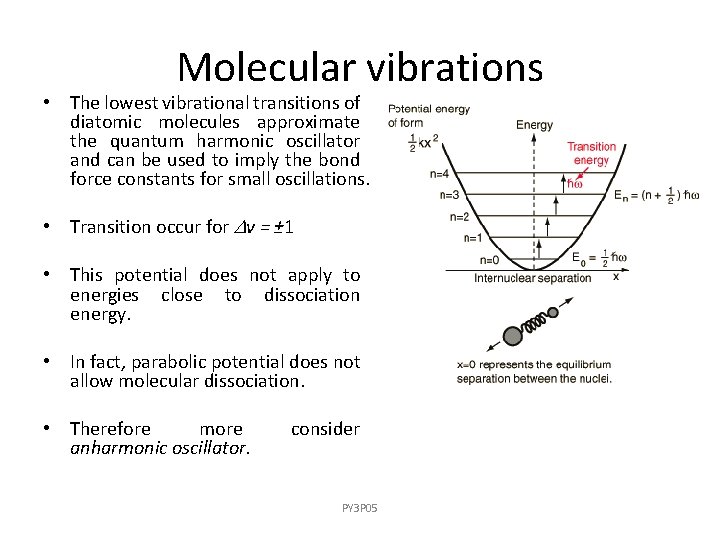
Molecular vibrations • The lowest vibrational transitions of diatomic molecules approximate the quantum harmonic oscillator and can be used to imply the bond force constants for small oscillations. • Transition occur for v = ± 1 • This potential does not apply to energies close to dissociation energy. • In fact, parabolic potential does not allow molecular dissociation. • Therefore more anharmonic oscillator. consider PY 3 P 05

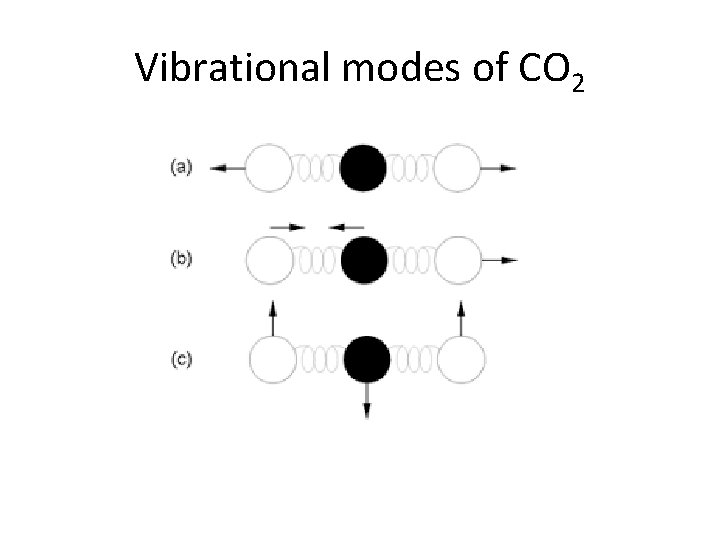
Vibrational modes of CO 2

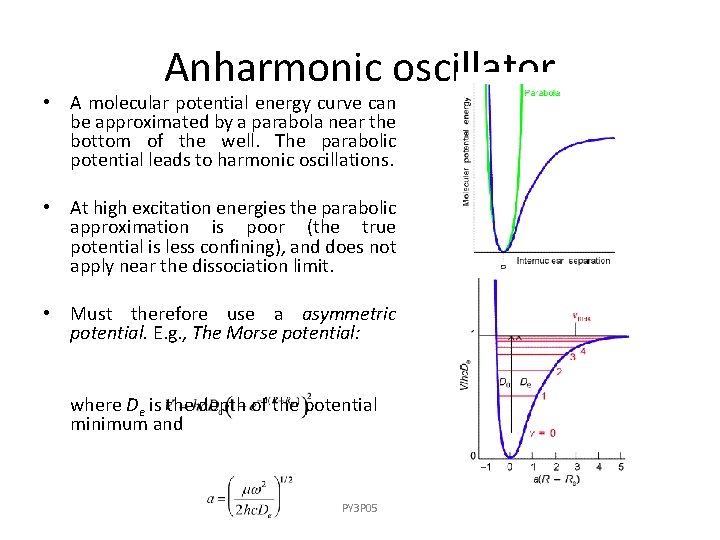
Anharmonic oscillator • A molecular potential energy curve can be approximated by a parabola near the bottom of the well. The parabolic potential leads to harmonic oscillations. • At high excitation energies the parabolic approximation is poor (the true potential is less confining), and does not apply near the dissociation limit. • Must therefore use a asymmetric potential. E. g. , The Morse potential: where De is the depth of the potential minimum and PY 3 P 05

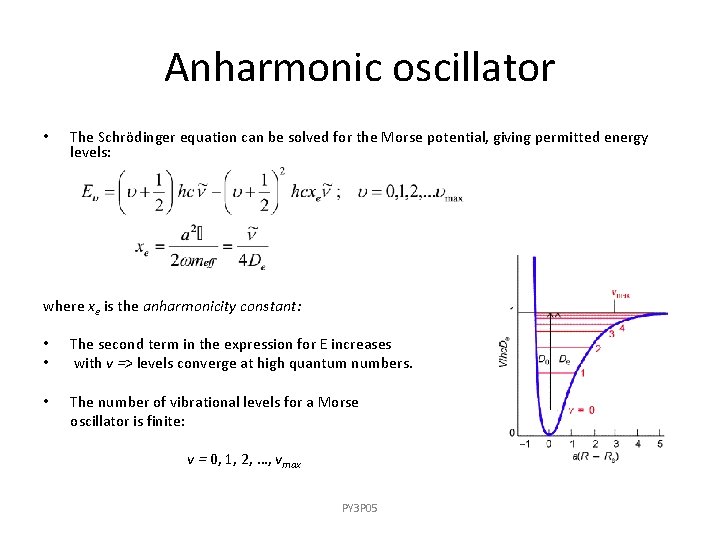
Anharmonic oscillator • The Schrödinger equation can be solved for the Morse potential, giving permitted energy levels: where xe is the anharmonicity constant: • • The second term in the expression for E increases with v => levels converge at high quantum numbers. • The number of vibrational levels for a Morse oscillator is finite: v = 0, 1, 2, …, vmax PY 3 P 05

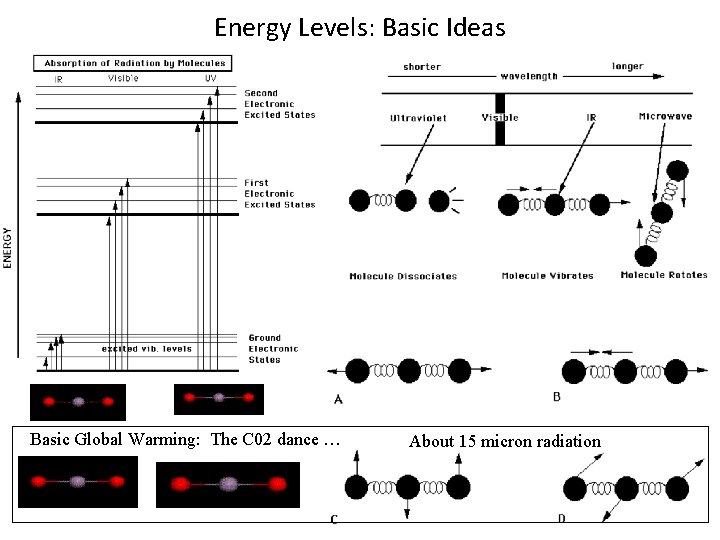
Energy Levels: Basic Ideas Basic Global Warming: The C 02 dance … About 15 micron radiation