Happy Fake Monday DO NOW n n Please





































- Slides: 50

Happy Fake Monday! DO NOW n n Please take out your homework (write 3 difficult test questions) and quiz your lab group. Have homework record labeled “ 3 test ? s” and ready to be stamped.


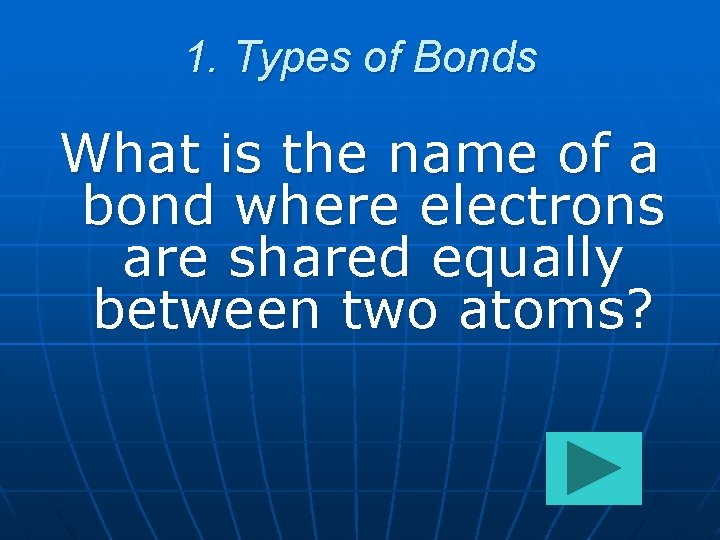
1. Types of Bonds What is the name of a bond where electrons are shared equally between two atoms?

1. Types of Bonds Nonpolar Covalent Bonds What is the name of a bond where electrons are shared equally between two atoms? 100

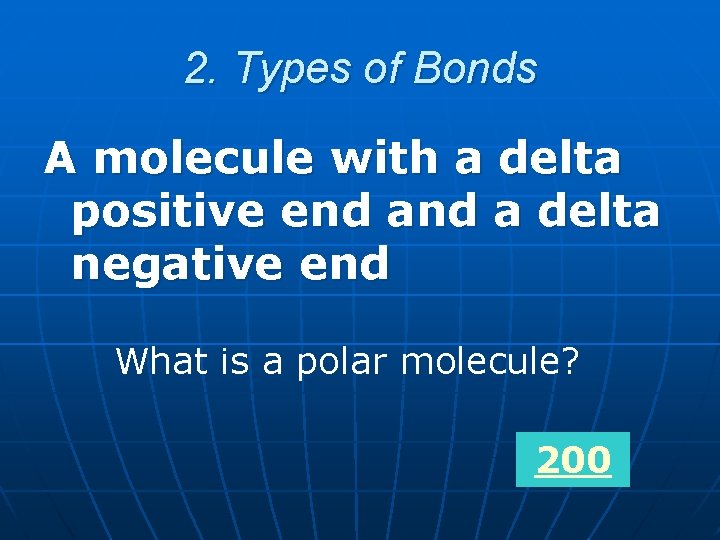
2. Types of Bonds What is a polar molecule?

2. Types of Bonds A molecule with a delta positive end a delta negative end What is a polar molecule? 200

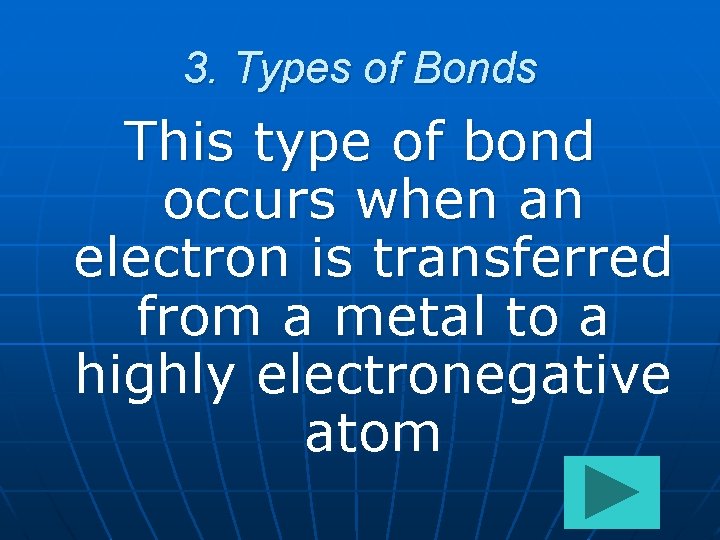
3. Types of Bonds This type of bond occurs when an electron is transferred from a metal to a highly electronegative atom

3. Types of Bonds Ionic Bond This type of bond occurs when an electron is transferred from a metal to a highly electronegative atom 300

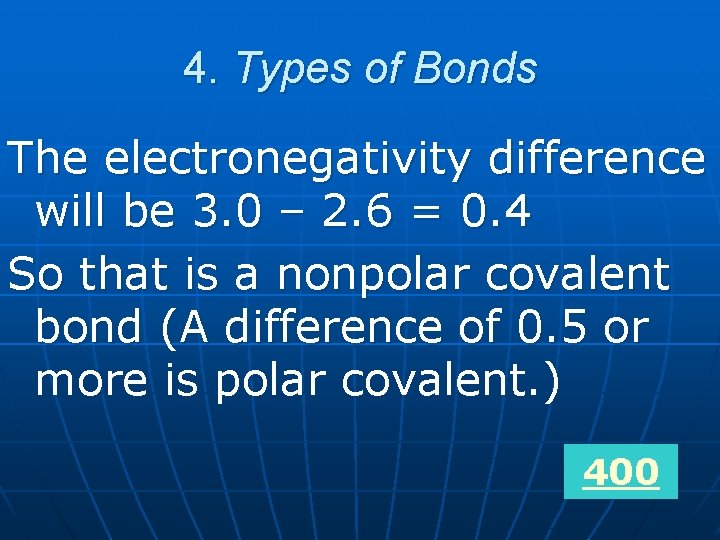
4. Types of Bonds n What type of bond would exist between atoms with electronegativity values of 3. 0 and 2. 6 ?

4. Types of Bonds The electronegativity difference will be 3. 0 – 2. 6 = 0. 4 So that is a nonpolar covalent bond (A difference of 0. 5 or more is polar covalent. ) 400

5. Types of Bonds Why does water have a much higher melting point than carbon dioxide even though carbon dioxide is heavier?

5. Types of Bonds Water is polar while CO 2 is nonpolar. It will take more energy to separate the water molecules from each other since the molecules are polar because the water molecules have a stronger attraction for one another. 500

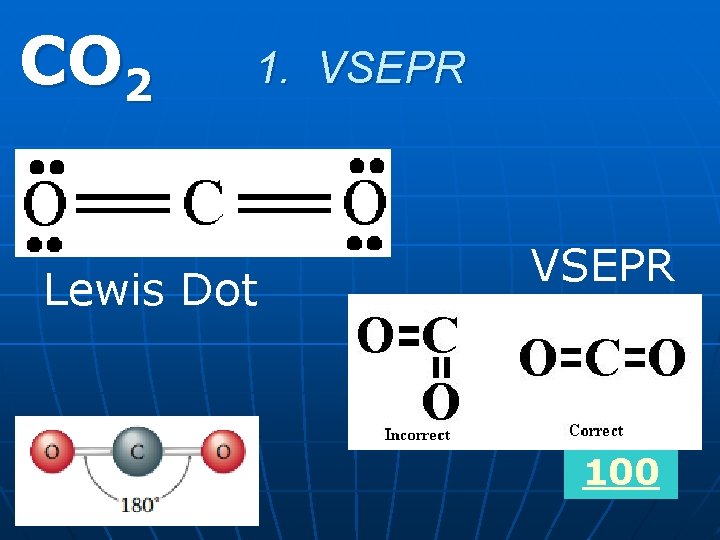
1. VSEPR Can you draw the Lewis Dot Structure AND VSEPR and write the formula of a molecule with a linear shape and a 180 degree bond angle?

CO 2 1. VSEPR Lewis Dot VSEPR 100

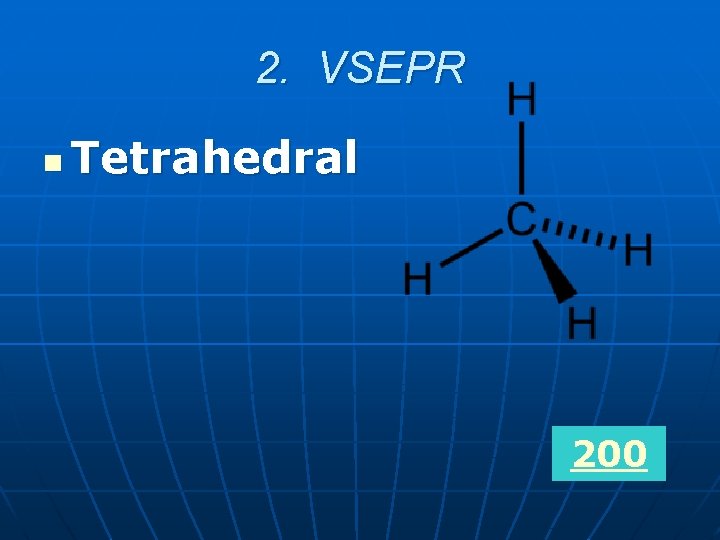
2. VSEPR What is the name of the shape of a compound with four atoms bonded to the central atom?

2. VSEPR n Tetrahedral 200

Write you wager on the white board.

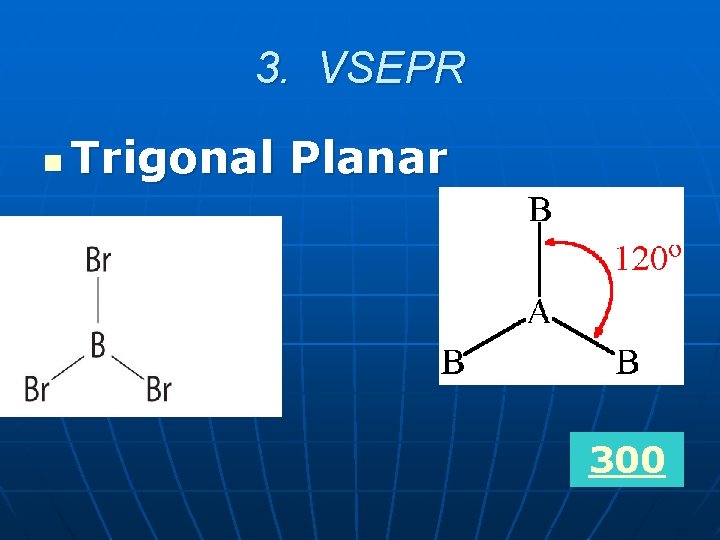
3. VSEPR What is the molecular shape of a molecule with 3 atoms bonded to the central atom and there are no unshared pairs of electrons?

3. VSEPR n Trigonal Planar 300

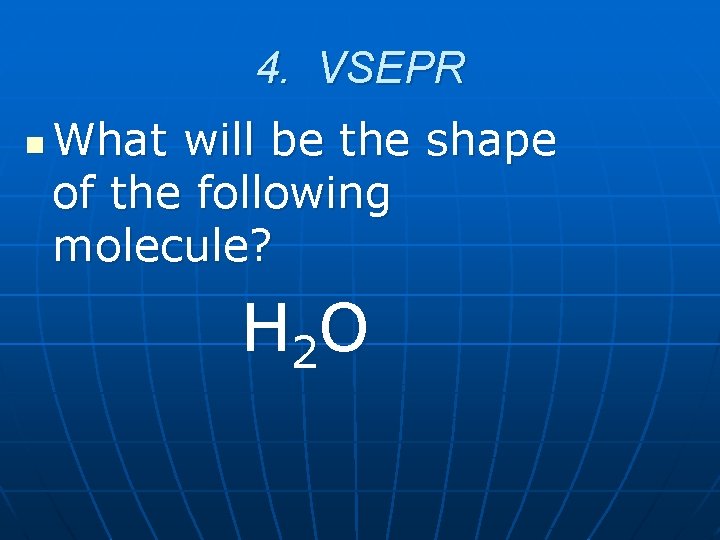
4. VSEPR n What will be the shape of the following molecule? H 2 O

4. VSEPR n Bent 400

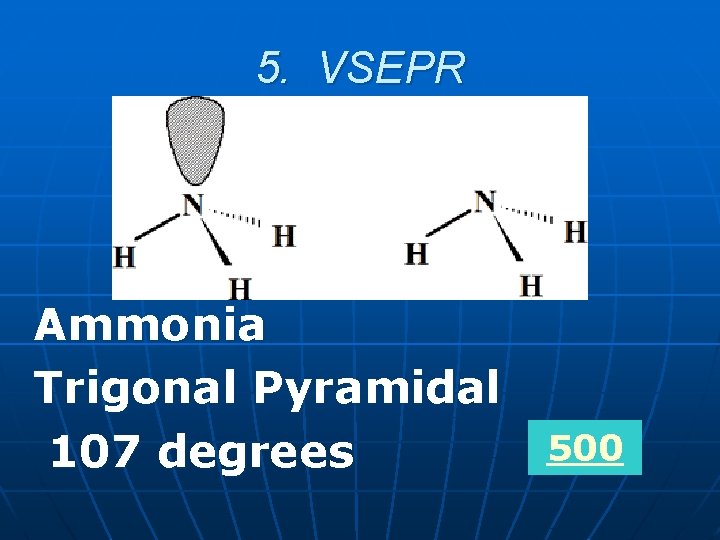
5. VSEPR What is the shape and bond angle for the following molecule? NH 3 Bonus 1: what’s the name of the molecule?

5. VSEPR Ammonia Trigonal Pyramidal 107 degrees 500

100 Compound Types n Which compounds have very low melting and boiling points?

100 Compound Types n Molecular compound 100

200 Compound Types n Type of Bonds that hold together a molecular compound

Compound Types n Covalent Bonds 200

300 Compound Types n Bronze, steel, and 18 k gold are examples of these.

Compound Types n Bronze, steel, and 18 k gold are examples of alloys. 300

1. Periodic Trends n As you move from left to right across a period, atomic radius tends to _______.

1. Periodic Trends n Decrease 100

2. Periodic Trends n What element has the highest electronegativity on the periodic table?

2. Periodic Trends F n flourine 200

Periodic Trends n Which element has the lowest ionization energy on the periodic table?

Periodic Trends Fr n 300

4. Periodic Trends n Of the following, which has the highest electronegativity? Mg, Al, Ca, Ga n

4. Periodic Trends n Of the following, which has the highest electronegativity? Mg, Al, Ca, Ga n

5. Periodic Trends n Draw all 3 periodic trends

5. Summary of Periodic Trends 500

Nomenclature What is the name of PH 3 ? n

Nomenclature What is the name of PH 3 ? n. Phosphorus trihydride n

Nomenclature What is the name of F 2 O ? n

Nomenclature What is the name of F 2 O ? n. Difluorine monoxide n

Nomenclature What is the formula for dinitrogen pentoxide? n

Nomenclature What is the formula for dinitrogen pentoxide? n N 2 O 5

Nomenclature What is the formula for xenon hexasulfide? n

Nomenclature What is the formula for xenon hexasulfide? Xe. S n 6

Nomenclature What is the name of S 2 O 3 ? n

Nomenclature What is the name of S 2 O 3 ? n. Disulfur trioxide n

Good luck on tomorrow’s exam! n Any additional questions? n Homework = please study! n