Hanjias Biochemistry Lecture Chapter 4 DNA Synthesis in

Hanjia’s Biochemistry Lecture Chapter 4 DNA Synthesis in vivo and in vitro David P. Clark Nanette Pazdernik 林翰佳 老師 課程網站 http: //lms. ls. ntou. edu. tw/course/106 hanjia@mail. ntou. edu. tw
Hanjia’s Biochemistry Lecture Outline • DNA synthesis in vivo – Please read textbook and review molecular biology • DNA synthesis in vitro – Chemical synthesis – Enzyme synthesis • Sequencing • PCR 2
Hanjia’s Biochemistry Lecture Chemical synthesis of DNA • • Origin from H. Dobind Khorana in 1970. No template! 3’ 5’ DNA synthesizer today is still use some origin idea • The limitation of DNA synthesizer is ~100 nucleotides in length. 3
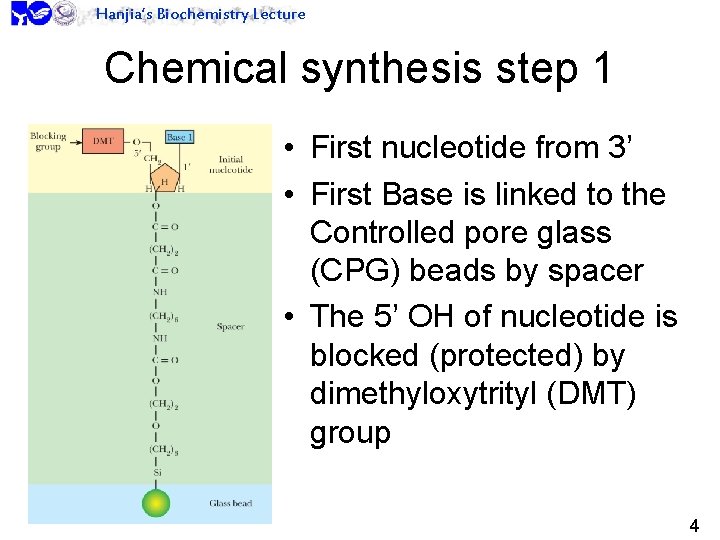
Hanjia’s Biochemistry Lecture Chemical synthesis step 1 • First nucleotide from 3’ • First Base is linked to the Controlled pore glass (CPG) beads by spacer • The 5’ OH of nucleotide is blocked (protected) by dimethyloxytrityl (DMT) group 4
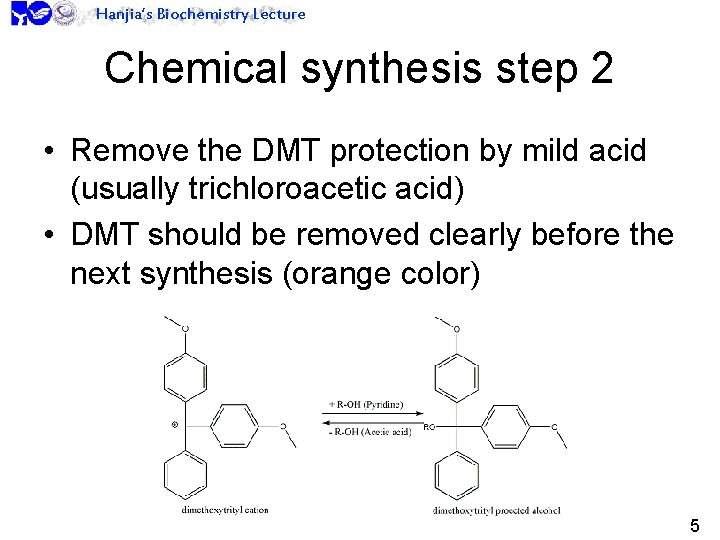
Hanjia’s Biochemistry Lecture Chemical synthesis step 2 • Remove the DMT protection by mild acid (usually trichloroacetic acid) • DMT should be removed clearly before the next synthesis (orange color) 5
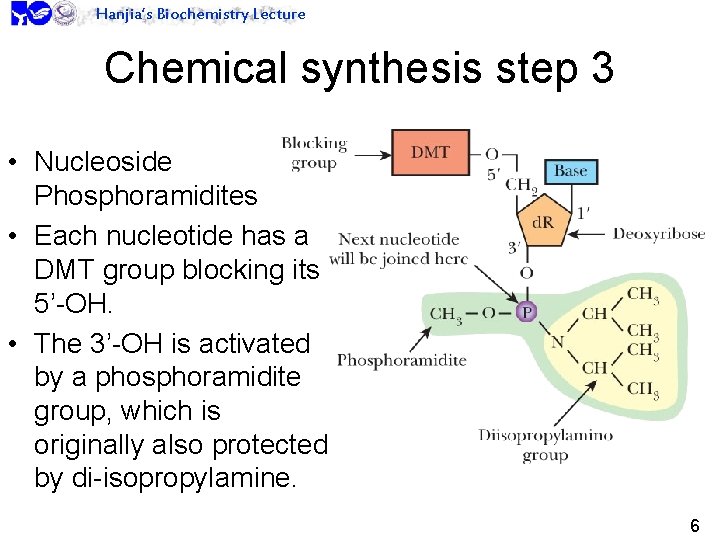
Hanjia’s Biochemistry Lecture Chemical synthesis step 3 • Nucleoside Phosphoramidites • Each nucleotide has a DMT group blocking its 5’-OH. • The 3’-OH is activated by a phosphoramidite group, which is originally also protected by di-isopropylamine. 6
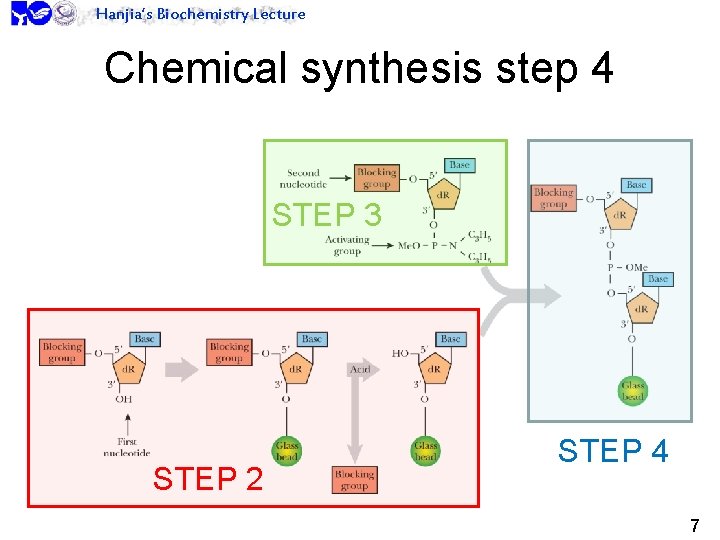
Hanjia’s Biochemistry Lecture Chemical synthesis step 4 STEP 3 STEP 2 STEP 4 7
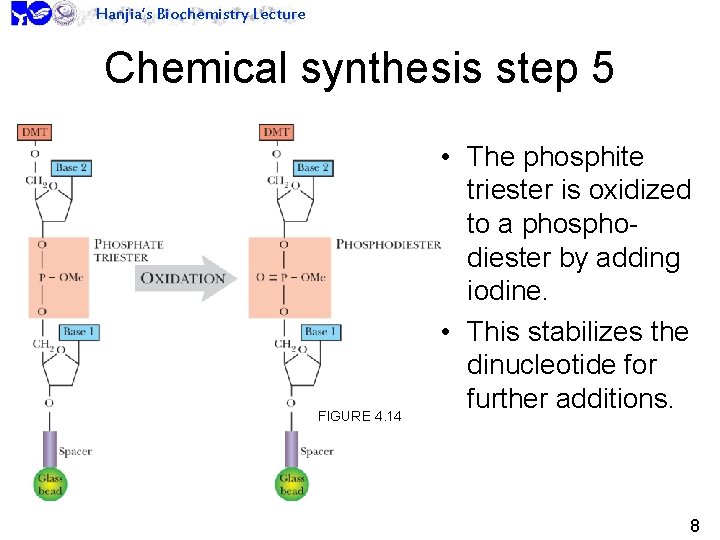
Hanjia’s Biochemistry Lecture Chemical synthesis step 5 FIGURE 4. 14 • The phosphite triester is oxidized to a phosphodiester by adding iodine. • This stabilizes the dinucleotide for further additions. 8
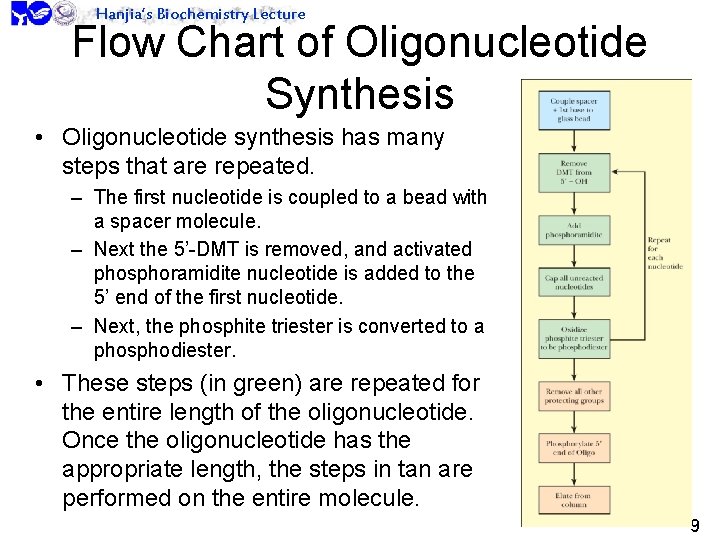
Hanjia’s Biochemistry Lecture Flow Chart of Oligonucleotide Synthesis • Oligonucleotide synthesis has many steps that are repeated. – The first nucleotide is coupled to a bead with a spacer molecule. – Next the 5’-DMT is removed, and activated phosphoramidite nucleotide is added to the 5’ end of the first nucleotide. – Next, the phosphite triester is converted to a phosphodiester. • These steps (in green) are repeated for the entire length of the oligonucleotide. Once the oligonucleotide has the appropriate length, the steps in tan are performed on the entire molecule. 9
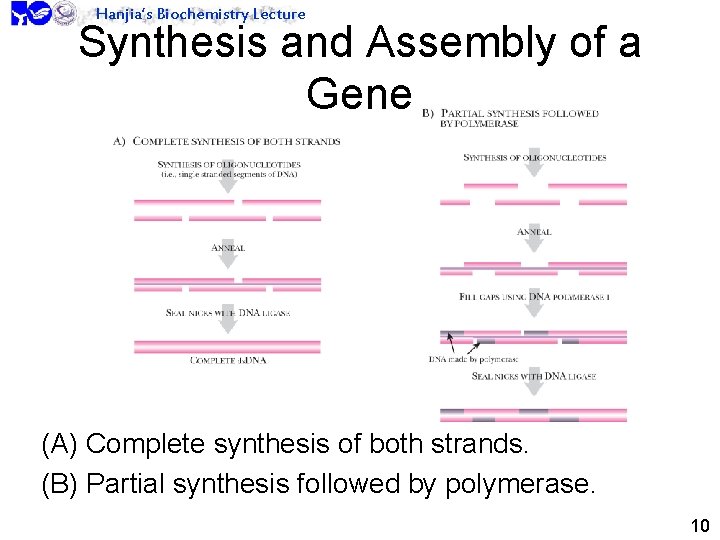
Hanjia’s Biochemistry Lecture Synthesis and Assembly of a Gene (A) Complete synthesis of both strands. (B) Partial synthesis followed by polymerase. 10
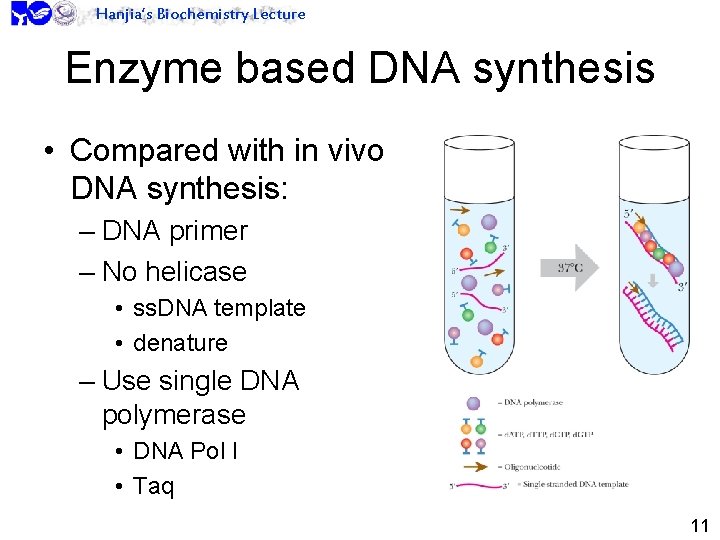
Hanjia’s Biochemistry Lecture Enzyme based DNA synthesis • Compared with in vivo DNA synthesis: – DNA primer – No helicase • ss. DNA template • denature – Use single DNA polymerase • DNA Pol I • Taq 11
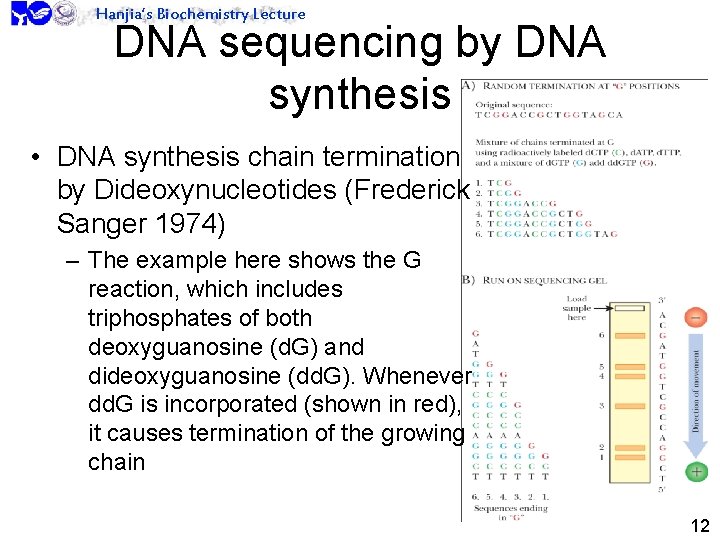
Hanjia’s Biochemistry Lecture DNA sequencing by DNA synthesis • DNA synthesis chain termination by Dideoxynucleotides (Frederick Sanger 1974) – The example here shows the G reaction, which includes triphosphates of both deoxyguanosine (d. G) and dideoxyguanosine (dd. G). Whenever dd. G is incorporated (shown in red), it causes termination of the growing chain 12
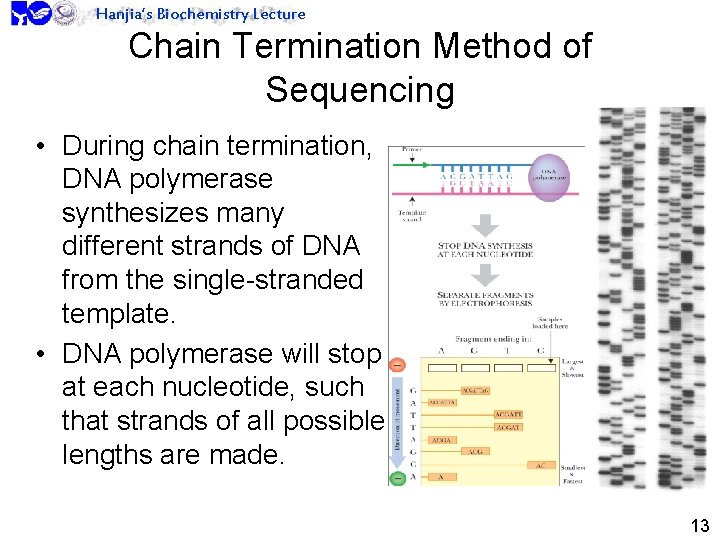
Hanjia’s Biochemistry Lecture Chain Termination Method of Sequencing • During chain termination, DNA polymerase synthesizes many different strands of DNA from the single-stranded template. • DNA polymerase will stop at each nucleotide, such that strands of all possible lengths are made. 13
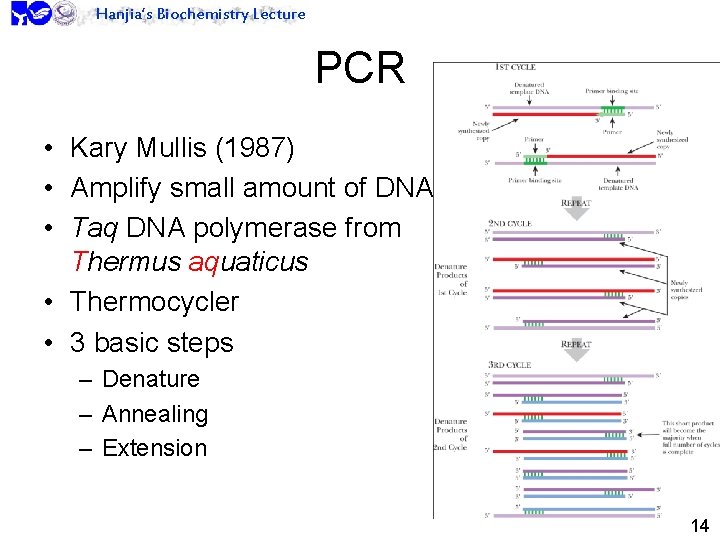
Hanjia’s Biochemistry Lecture PCR • Kary Mullis (1987) • Amplify small amount of DNA • Taq DNA polymerase from Thermus aquaticus • Thermocycler • 3 basic steps – Denature – Annealing – Extension 14
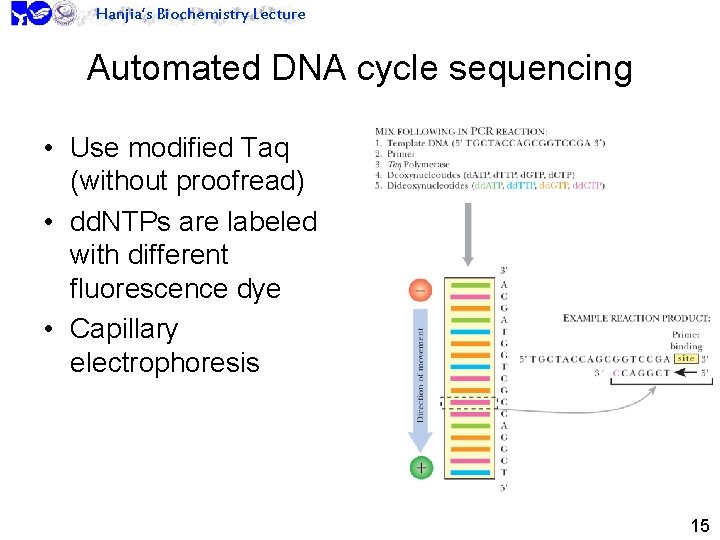
Hanjia’s Biochemistry Lecture Automated DNA cycle sequencing • Use modified Taq (without proofread) • dd. NTPs are labeled with different fluorescence dye • Capillary electrophoresis 15
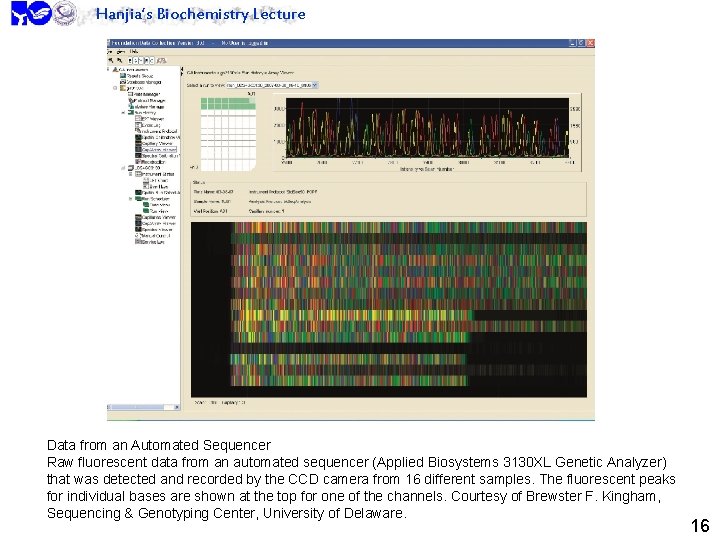
Hanjia’s Biochemistry Lecture Data from an Automated Sequencer Raw fluorescent data from an automated sequencer (Applied Biosystems 3130 XL Genetic Analyzer) that was detected and recorded by the CCD camera from 16 different samples. The fluorescent peaks for individual bases are shown at the top for one of the channels. Courtesy of Brewster F. Kingham, Sequencing & Genotyping Center, University of Delaware. 16
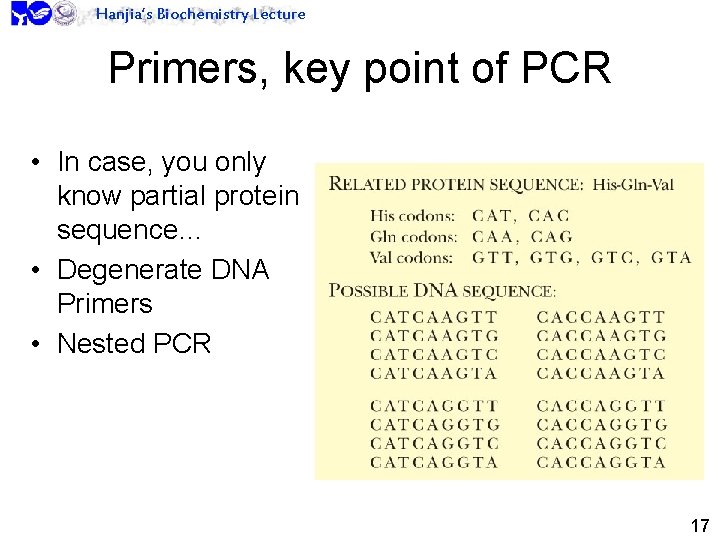
Hanjia’s Biochemistry Lecture Primers, key point of PCR • In case, you only know partial protein sequence… • Degenerate DNA Primers • Nested PCR 17
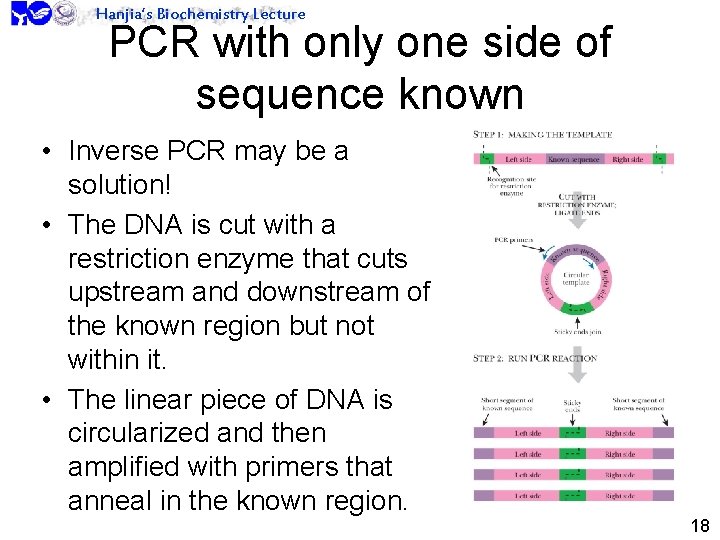
Hanjia’s Biochemistry Lecture PCR with only one side of sequence known • Inverse PCR may be a solution! • The DNA is cut with a restriction enzyme that cuts upstream and downstream of the known region but not within it. • The linear piece of DNA is circularized and then amplified with primers that anneal in the known region. 18
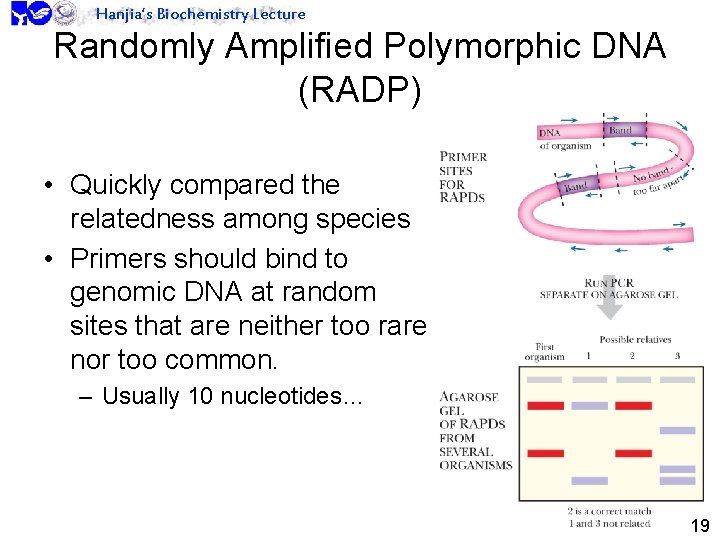
Hanjia’s Biochemistry Lecture Randomly Amplified Polymorphic DNA (RADP) • Quickly compared the relatedness among species • Primers should bind to genomic DNA at random sites that are neither too rare nor too common. – Usually 10 nucleotides… 19
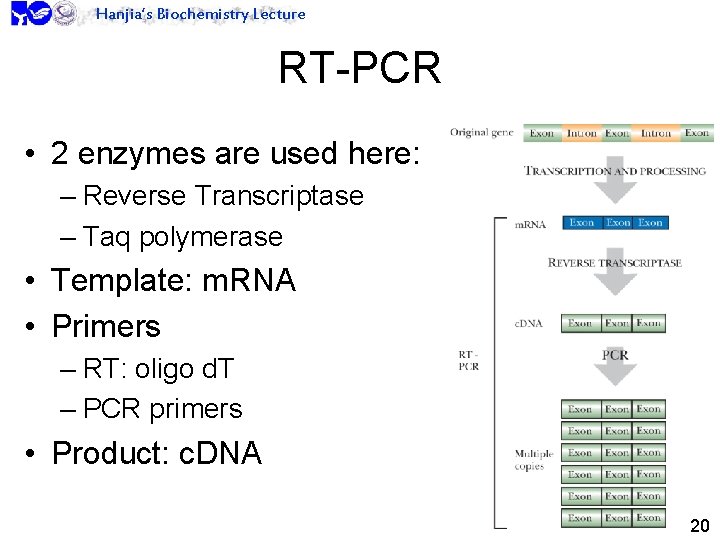
Hanjia’s Biochemistry Lecture RT-PCR • 2 enzymes are used here: – Reverse Transcriptase – Taq polymerase • Template: m. RNA • Primers – RT: oligo d. T – PCR primers • Product: c. DNA 20
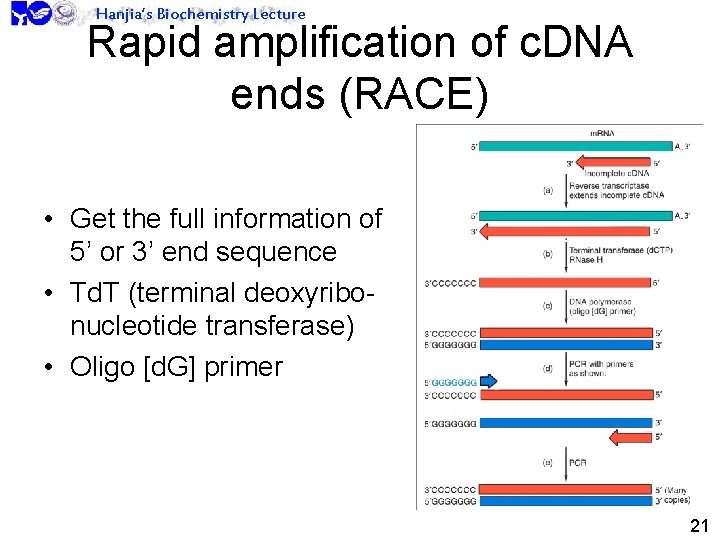
Hanjia’s Biochemistry Lecture Rapid amplification of c. DNA ends (RACE) • Get the full information of 5’ or 3’ end sequence • Td. T (terminal deoxyribonucleotide transferase) • Oligo [d. G] primer 21
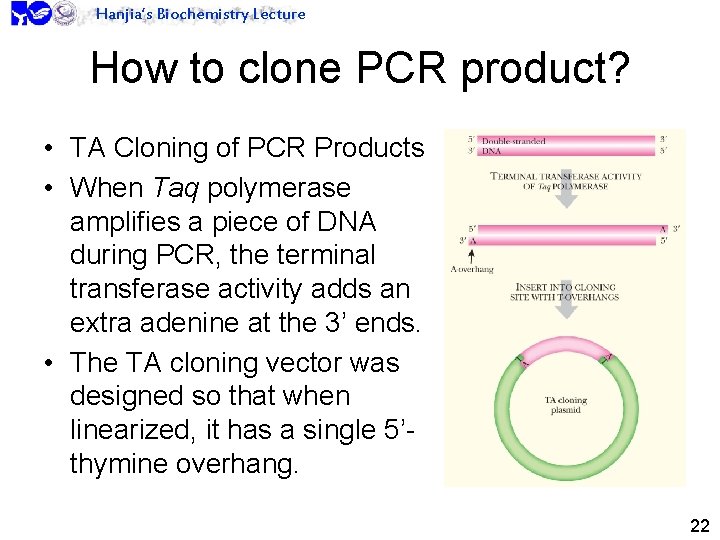
Hanjia’s Biochemistry Lecture How to clone PCR product? • TA Cloning of PCR Products • When Taq polymerase amplifies a piece of DNA during PCR, the terminal transferase activity adds an extra adenine at the 3’ ends. • The TA cloning vector was designed so that when linearized, it has a single 5’thymine overhang. 22
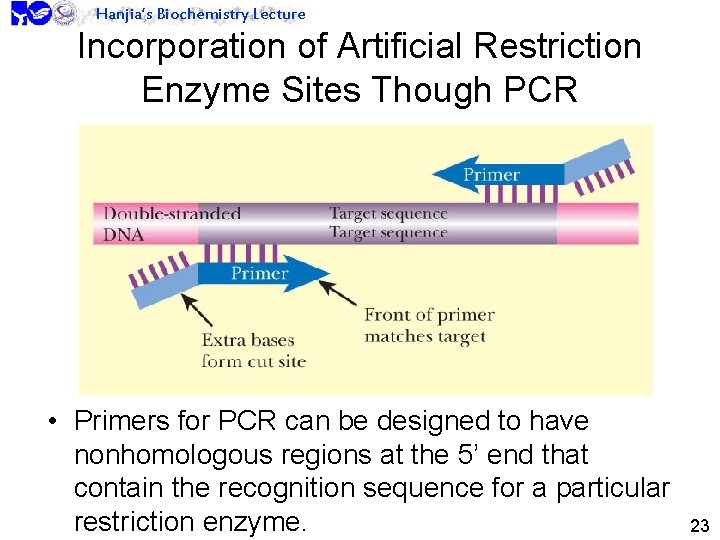
Hanjia’s Biochemistry Lecture Incorporation of Artificial Restriction Enzyme Sites Though PCR • Primers for PCR can be designed to have nonhomologous regions at the 5’ end that contain the recognition sequence for a particular restriction enzyme. 23
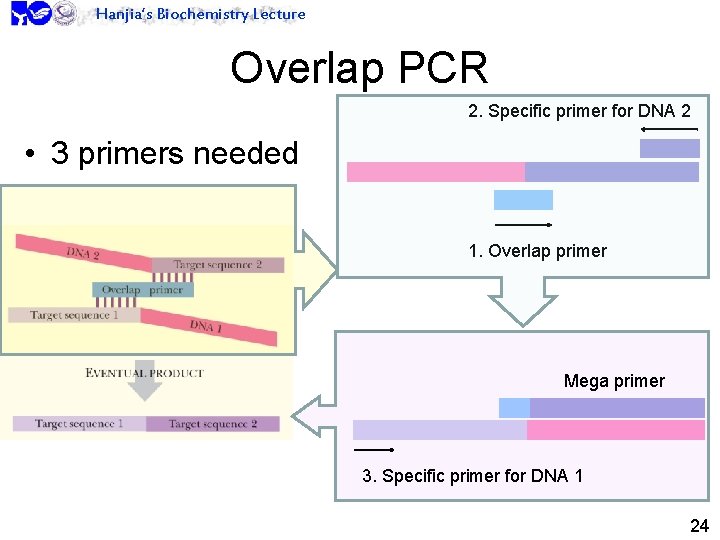
Hanjia’s Biochemistry Lecture Overlap PCR 2. Specific primer for DNA 2 • 3 primers needed 1. Overlap primer Mega primer 3. Specific primer for DNA 1 24
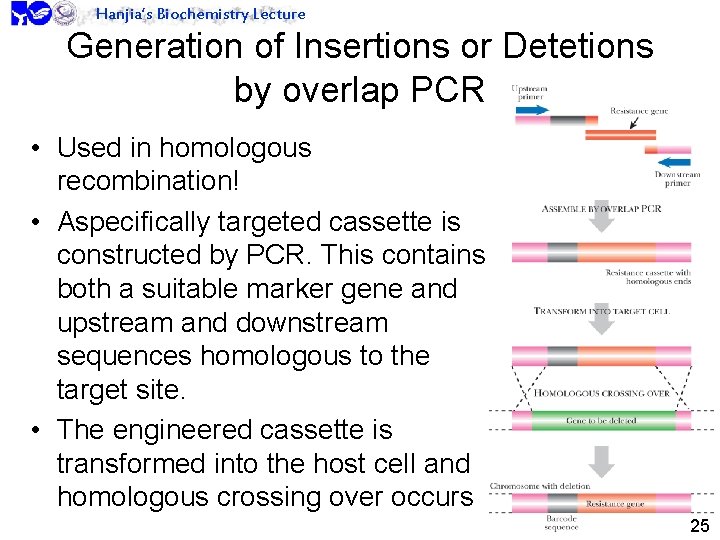
Hanjia’s Biochemistry Lecture Generation of Insertions or Detetions by overlap PCR • Used in homologous recombination! • Aspecifically targeted cassette is constructed by PCR. This contains both a suitable marker gene and upstream and downstream sequences homologous to the target site. • The engineered cassette is transformed into the host cell and homologous crossing over occurs 25
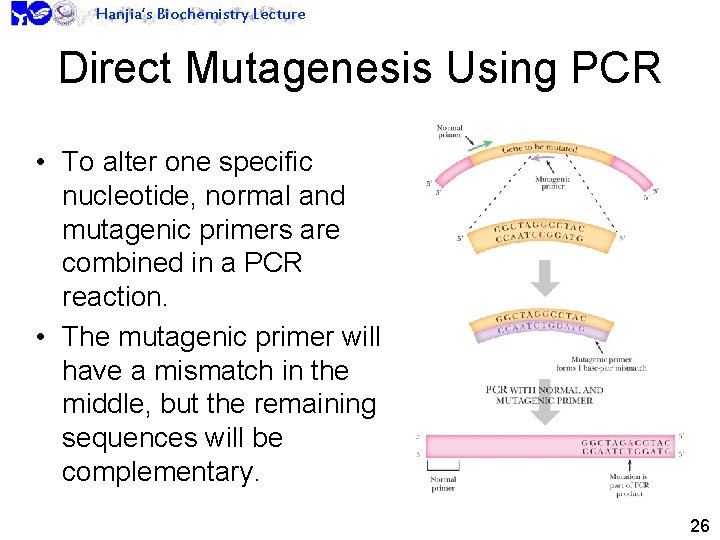
Hanjia’s Biochemistry Lecture Direct Mutagenesis Using PCR • To alter one specific nucleotide, normal and mutagenic primers are combined in a PCR reaction. • The mutagenic primer will have a mismatch in the middle, but the remaining sequences will be complementary. 26
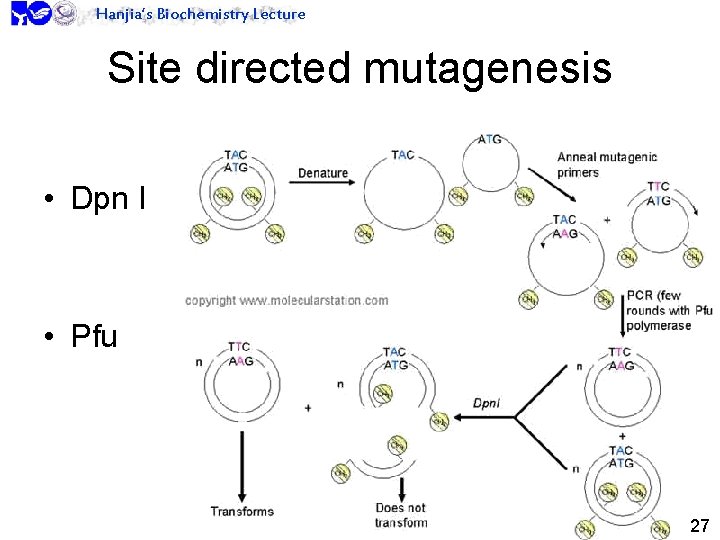
Hanjia’s Biochemistry Lecture Site directed mutagenesis • Dpn I • Pfu 27
- Slides: 27