Handout Please pick up a copy of course




















![DNA is synthesized in a 5’ to 3’ direction [Fig. 6 -10 (p. 202)] DNA is synthesized in a 5’ to 3’ direction [Fig. 6 -10 (p. 202)]](https://slidetodoc.com/presentation_image_h/f9b25fa94101b216ab47b66dda1e92b4/image-46.jpg)

- Slides: 47

Handout • Please pick up a copy of course outline, lecture# 1 handout and a blank card from the front • On the card, write your name, student number, contact info (telephone number, e-mail address) and your expectations from the course • Return the information card to me at the end of the lecture

CELL BIOLOGY 1 (BIOL 200) (2006 S): COURSE OUTLINE • INSTRUCTOR Dr. Santokh Singh Department of Botany Bioscience Bldg. # 2528 (Second Floor) Telephone: 604 -822 -3330, E-mail: santokh@interchange. ubc. ca OFFICE HOURS: during lecture breaks or by appointment

TUTORIAL COORDINATOR AND TAs • TUTORIAL COORDINATOR Jamie Pighin, Dept. Botany, E-mail: jpighin 2002@yahoo. ca • TEACHING ASSISTANTS Apurva Bhargava, E-mail: apurv@interchange. ubc. ca Jacqueline Monaghan, E-mail: monagh 9@interchange. ubc. ca

PRE-REQUISITE COURSES • All of BIOL 112 and one of CHEM 123 or CHEM 113; or all of • SCIE 1, or all of BIOL 121 and co-requisite of all of CHEM 203

TEXTBOOK Essential Cell Biology Eds. B. Alberts et al. Garland Publishing, 2 nd Edition, 2004.

COURSE CONTENT Nine Units: 1. 2. 3. 4. 5. 6. 7. 8. 9. Introduction of Cell Structure & Function Informational Macromolecules Interphase nucleus Biological Information Flow (Transcription & Translation) Structure & Function of Membranes Mitochondria & Chloroplasts Endomembranes Cytoskeleton Cell Cycle

TEACHING & LEARNING • Lecture outlines; powerpoint slides; textbook; tutorials • Course website URL: https: //www. botany. ubc. ca/biol 200/

COURSE EVALUATION • Tutorials: 25% • Mid-term examination: 25% • Final Examination: 50%

EXAMINATION DATES • Midterm Examination (in class): June 29 (1 - 1: 50 PM) • Final Examination (in class): July 7 (1 - 3: 30 PM)

POLICIES • A student must pass the lecture component to pass the course. The maximum grade obtainable by students failing the lecture portion of the course is 45% • An individual student's marks for each of the three components, tutorial grade, midterm exam and final exam will be counted (No grades will be dropped) • Both midterm and final exams may contain short answer, multiple choice, fill-in the blanks, definitions, experimental, and essay questions • Final exam will be cumulative: All lecture and tutorial material may be covered • If you miss the final exam you must contact the office of the Dean of Science as soon as possible



Objectives for BIOL 200 • Have a functional grasp of the structure and function of all major eukaryotic cell systems • Understand how these systems interact in the execution of complex processes, i. e. secretion or cell division • Know the basic experimental techniques employed in cell biology research • Facilitate the integration of concepts and data from many subsequent courses e. g. biochemistry, genetics and physiology

Course approach • Presentation of material with emphasis on basic points • Provision of specific details on tricky points • Discussion of key concepts and their experimental basis • Discussion of specific types of exam questions

BIOL 200 (Section 921) Lecture # 1; June 19, 2006 • Reading: Essential Cell Biology (ECB) 2 nd edition. Chap 1 and Chap 2, Chap 5, pages 169 -177 Background Study Material: Study Panels 2 -1, 2 -2, 2 -3, 2 -4, 2 -5, 2 -6, 2 -7 (pages 66 -79). • • Good questions: 1 -6, 1 -7, 1 -9, 1 -10, 1 -12, 1 -18 Assignments: 1. Make a diagram relating the relative size of a typical nucleus, mitochondrion, a bacterium and a ribosome. 2. Practice to identify the cell organelles in electron micrographs of a variety of cell types using the Image Database website: http: //www. biomedia. cellbiology. ubc. ca/ • 1. 2. 3. Learning Objectives Be able to critically define cells and organelles Know the major classes of eukaryotic cell organelles and their functions. Develop a general feel for the flow of information and the flow of material in cells. 4. Know the different types of microscopy and their functions 5. Know the mechanism and key reactions of synthesis of macromolecules 6. Know the different forces that stabilize the DNA structure

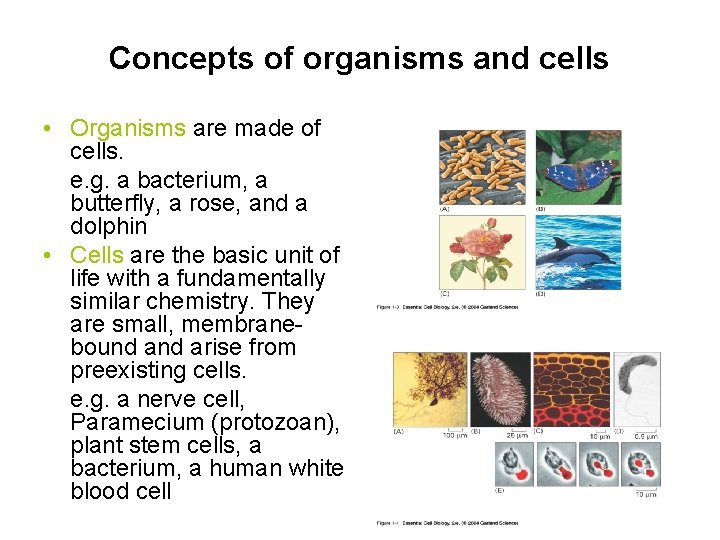
Concepts of organisms and cells • Organisms are made of cells. e. g. a bacterium, a butterfly, a rose, and a dolphin • Cells are the basic unit of life with a fundamentally similar chemistry. They are small, membranebound arise from preexisting cells. e. g. a nerve cell, Paramecium (protozoan), plant stem cells, a bacterium, a human white blood cell

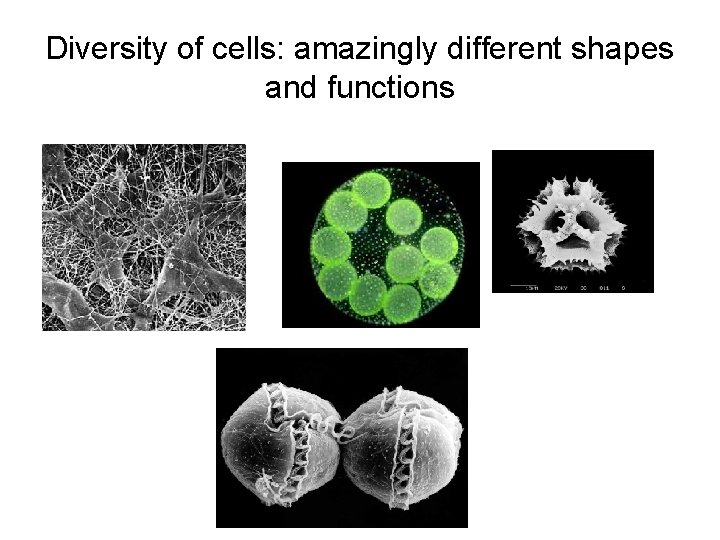
Diversity of cells: amazingly different shapes and functions

Unity of cells • • • Living cells have a similar basic chemistry and information flow Cells have evolved from same ancestors Cells from different species share similar genes (e. g. defects in the gene “Kit” result in white patches on foreheads of both the human baby and the mouse.

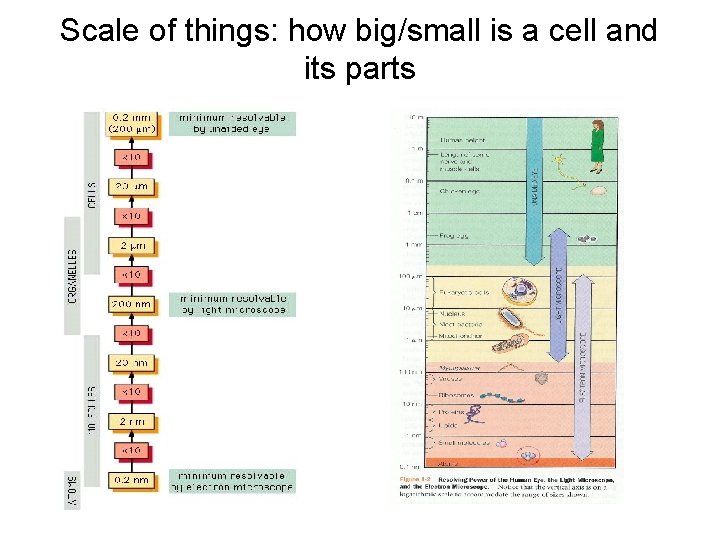
Scale of things: how big/small is a cell and its parts

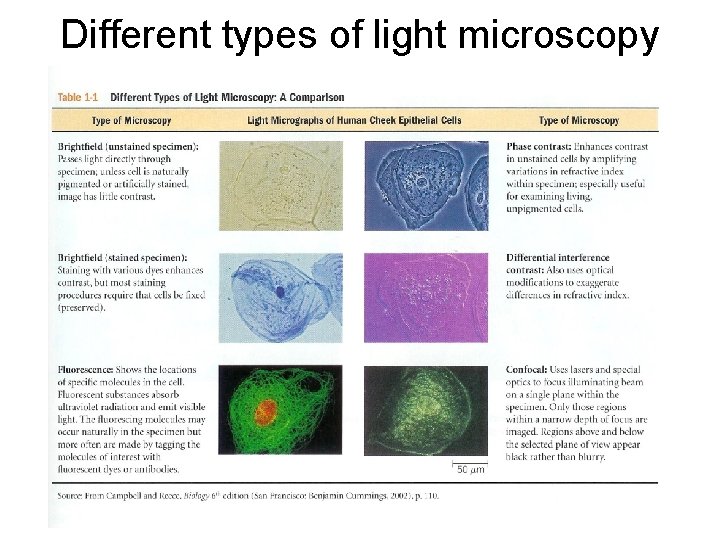
Light microscopy • Light microscopy involves visible light and glass lenses to form an image of the specimen. • Advantages: Live cells can be viewed. Images show color if specimens are stained with dyes. Disadvantages: Limit of resolution is 0. 2 um.

Specialized types of light microscopy • Phase contrast and differential interference contrast microscopy – enhance and amplify variations in refractive index within specimen. • Fluorescence microscopy - uses fluorescent dyes and antibodies to bind to and detect certain proteins or other molecules. These fluorescent molecules absorb ultraviolet light and emit light of a lower wavelength. Fluorescently labeled cells glow bright against a dark background. • Confocal scanning light microscopy (CCLM) -type of fluorescent microscope: uses a laser beam to excite the fluorochrome in a thin slice called a Z-section or optical section. The light emitted by the fluorochrome is focussed on a site, i. e. "confocal", with a pinhole aperture that removes out of focus fluorescence. Allows many slices of a structure to be gathered as digital image files and then 3 -D reconstructions made on the computer.

Different types of light microscopy

Transmission electron microscopy • Transmission electron microscopy (TEM) is highly analogous to light microscope. Beam of electrons go through thin layer of sample and are either diffracted by interacting with sample or go straight through (transmitted). Resolution of the electron microscope about 0. 2 nm Pros: good resolution of size range important to cells (200 nm to 0. 2 nm; size from organelles to macromolecules). Cons: samples must be able to withstand electron bombardment and vacuum, so elaborate sample preparation is necessary (fixation, resin embedding, sectioning into slices 50 -100 nm, heavy metal stain). Hard to reconstruct 3 -D structure from 2 -D slices. Fig. 1 -8: TEM of a thin section of a liver cell

Scanning electron microscopy • Scanning electron microscopy (SEM)-beam of electrons is scanned across a sample and as it hits the sample, secondary electrons are ejected. These are collected by a secondary electron detector which electronically builds an image based on the electron intensity (from white to black). Pros: great for surfaces, 3 -D images Cons: electrons require vacuum so most samples have to be fixed and dried. From: Becker et al. World of the Cell

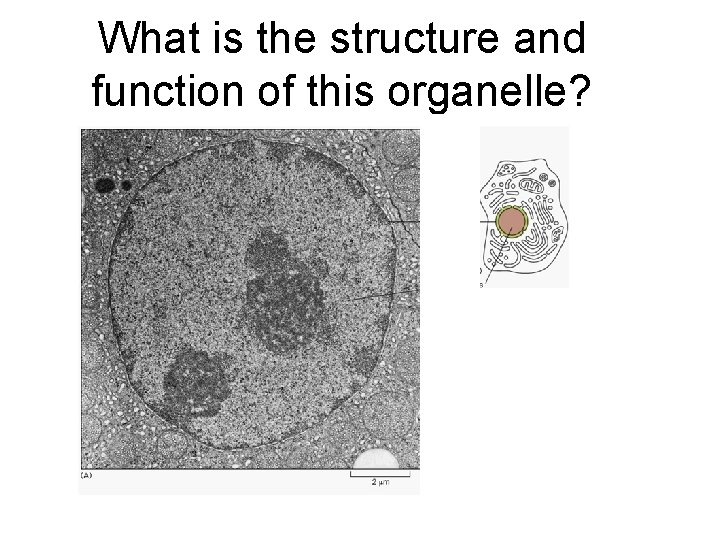
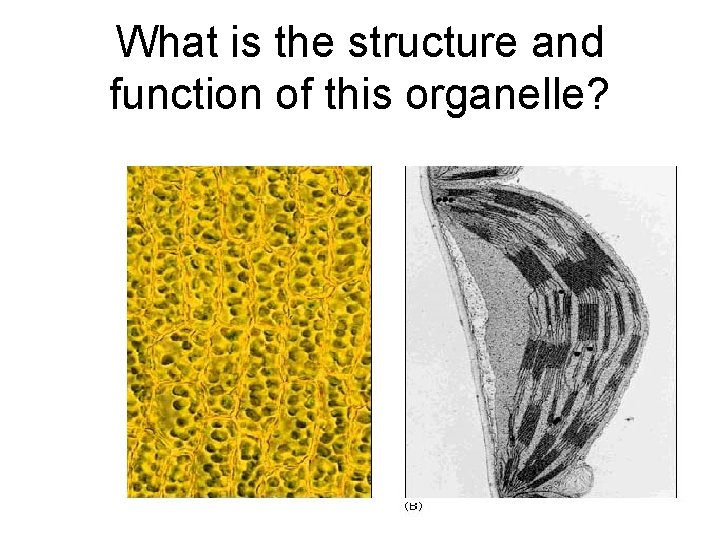
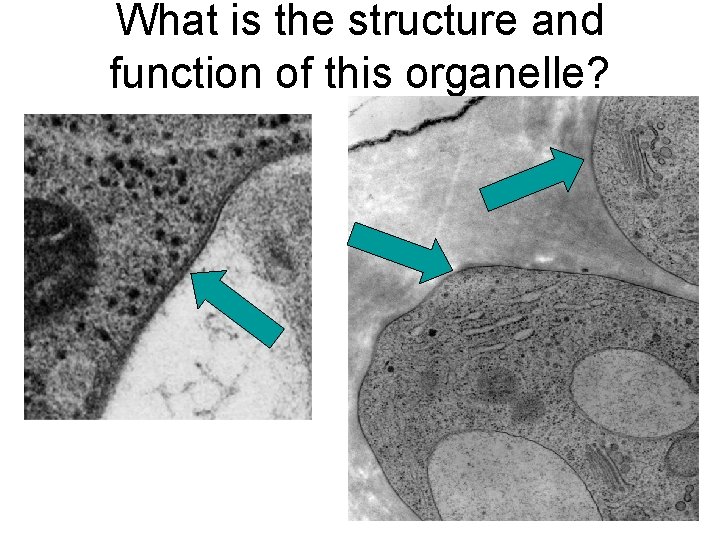
What is the structure and function of this organelle?

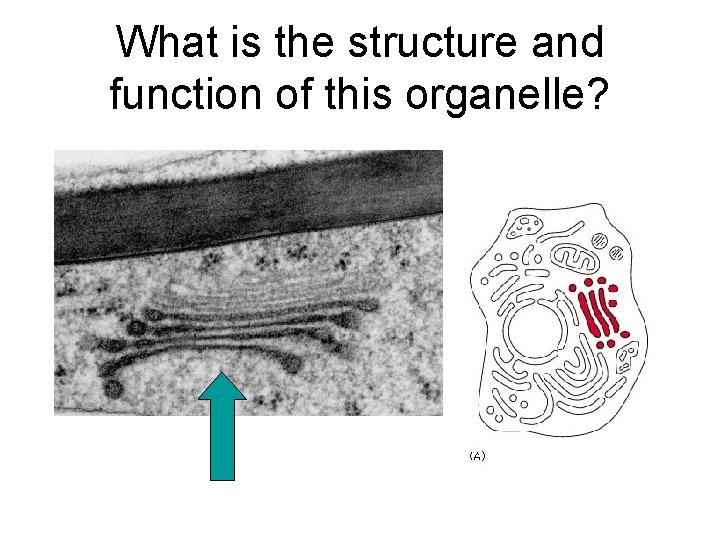
What is the structure and function of this organelle?

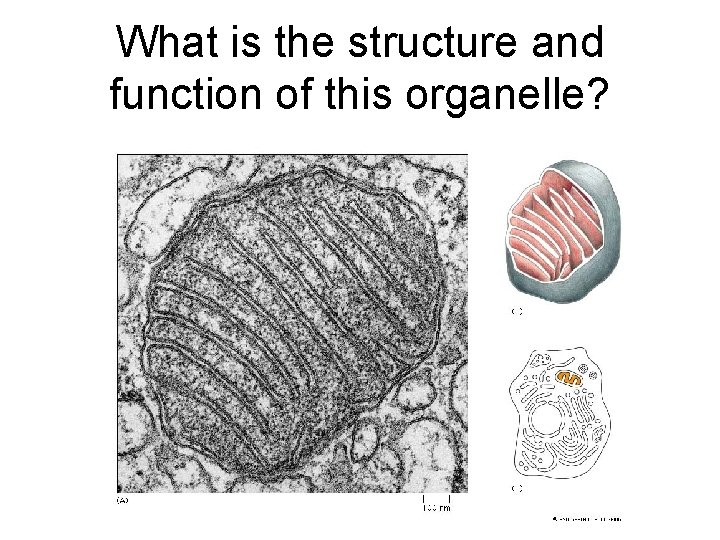
What is the structure and function of this organelle?

What is the structure and function of this organelle?

What is the structure and function of this organelle?

What is the structure and function of this organelle?

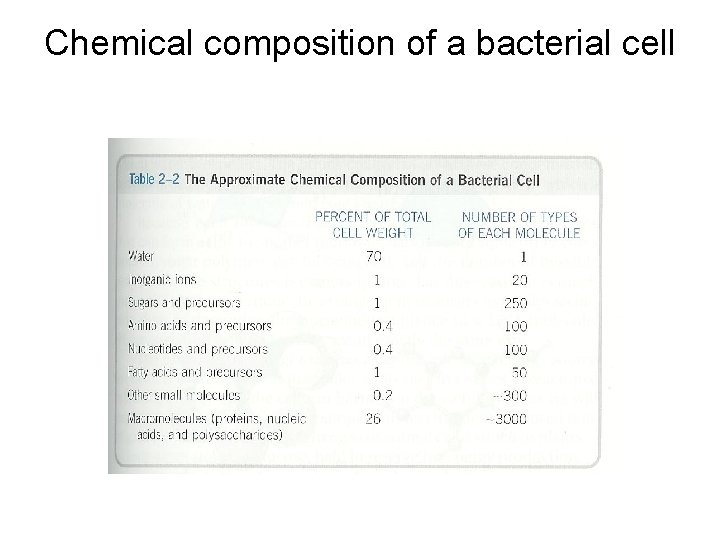
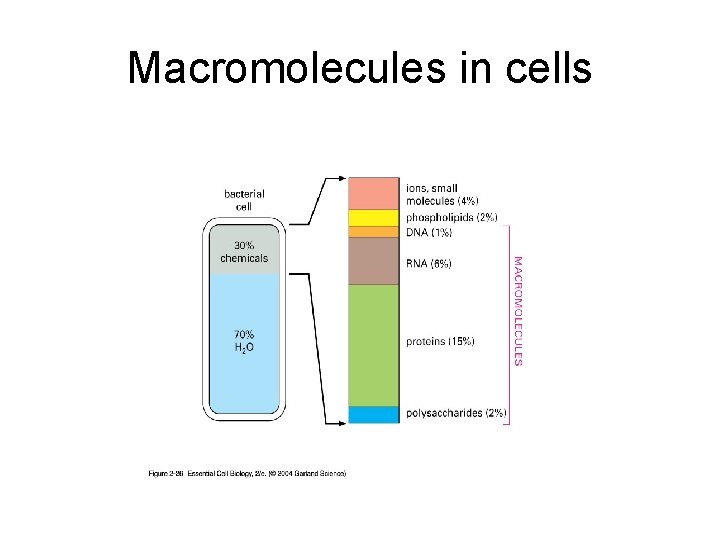
Chemical composition of a bacterial cell

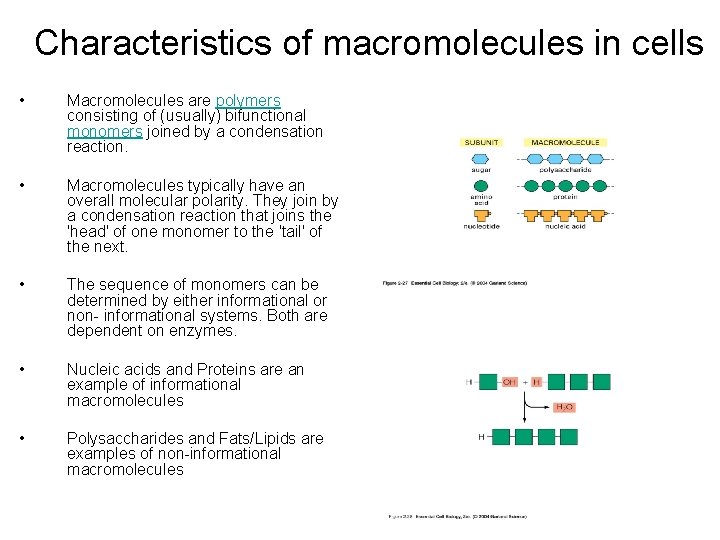
Characteristics of macromolecules in cells • Macromolecules are polymers consisting of (usually) bifunctional monomers joined by a condensation reaction. • Macromolecules typically have an overall molecular polarity. They join by a condensation reaction that joins the 'head' of one monomer to the 'tail' of the next. • The sequence of monomers can be determined by either informational or non- informational systems. Both are dependent on enzymes. • Nucleic acids and Proteins are an example of informational macromolecules • Polysaccharides and Fats/Lipids are examples of non-informational macromolecules

Macromolecules in cells

Macromolecules and their monomeric subunits

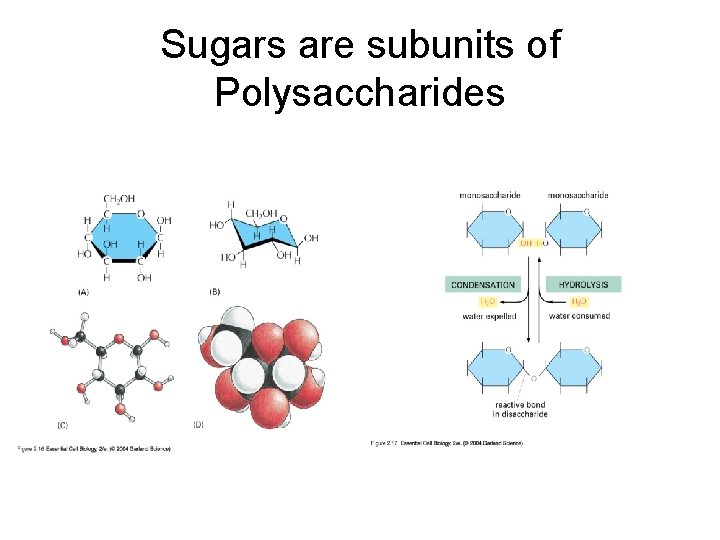
Sugars are subunits of Polysaccharides

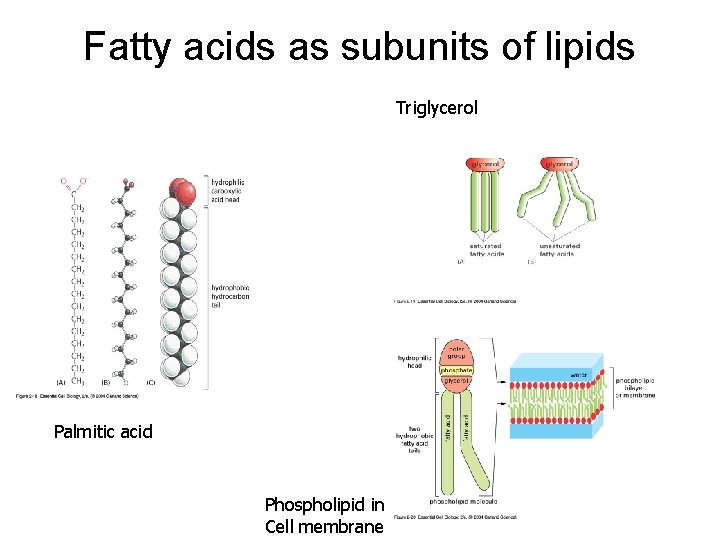
Fatty acids as subunits of lipids Triglycerol Palmitic acid Phospholipid in Cell membrane

Amino acids are the subunits of proteins

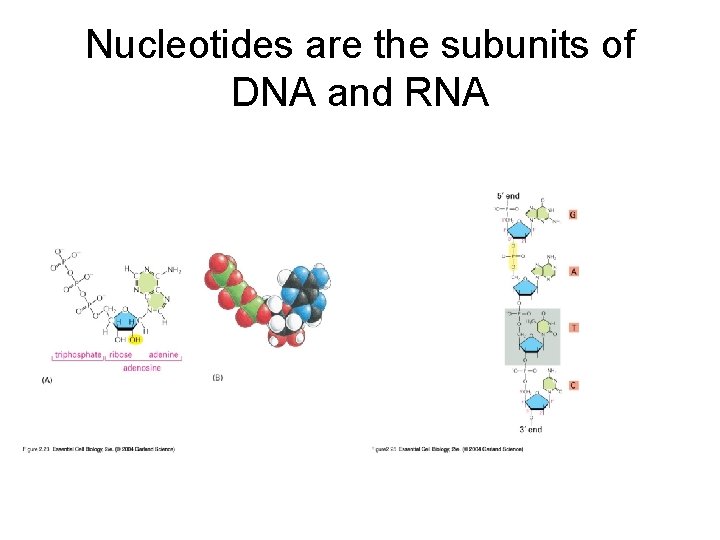
Nucleotides are the subunits of DNA and RNA

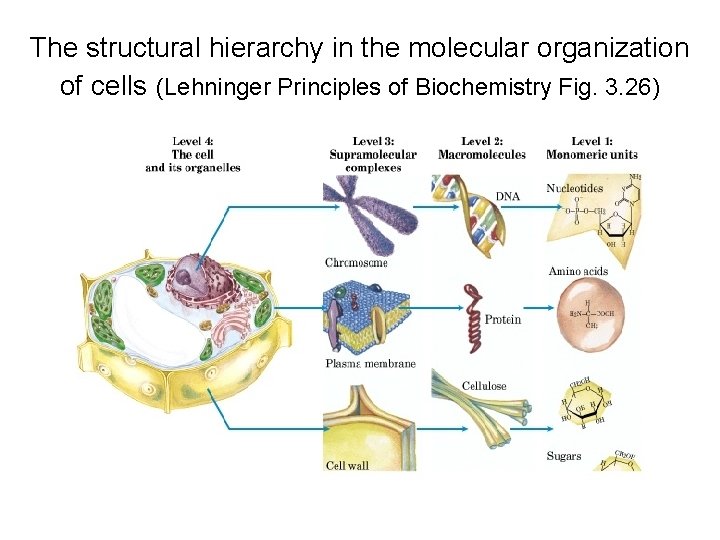
The structural hierarchy in the molecular organization of cells (Lehninger Principles of Biochemistry Fig. 3. 26)

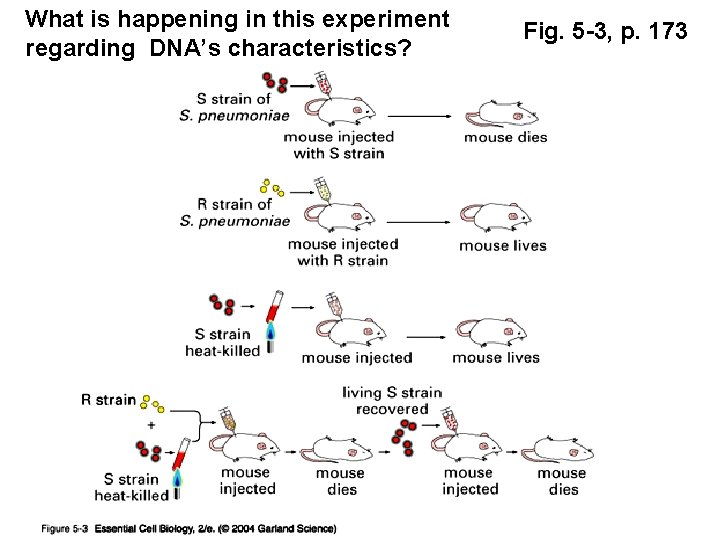
What is happening in this experiment regarding DNA’s characteristics? Fig. 5 -3, p. 173

Fig. 5 -4: DNA is genetic material.

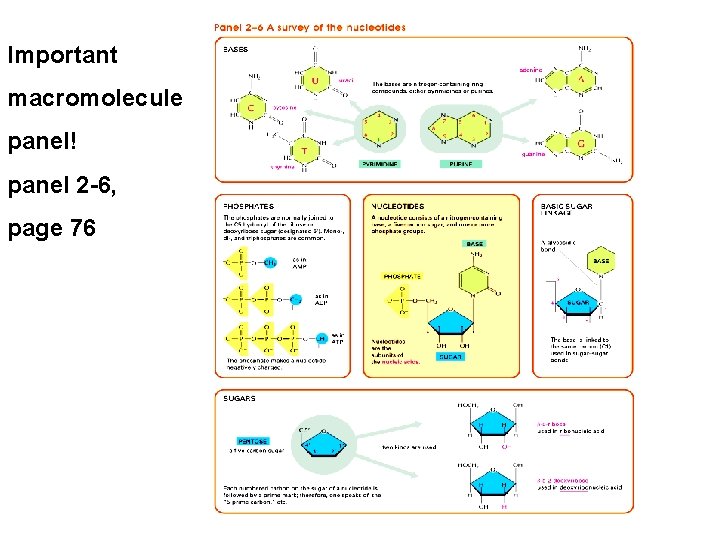
Important macromolecule panel! panel 2 -6, page 76

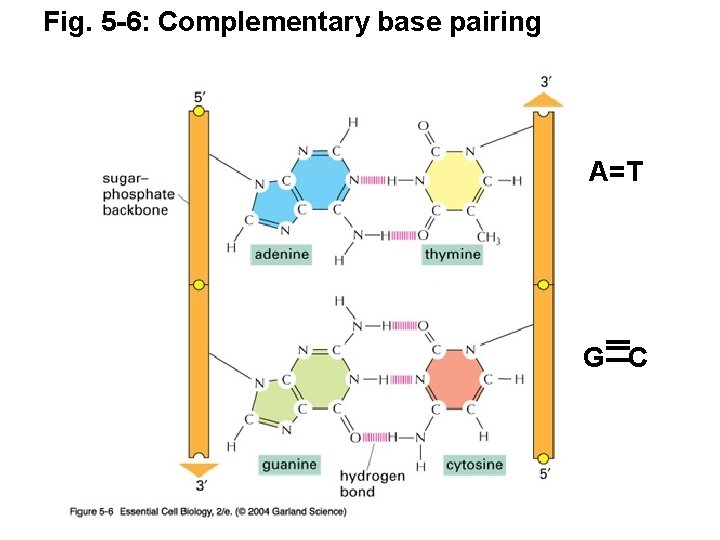
Fig. 5 -6: Complementary base pairing A=T G C

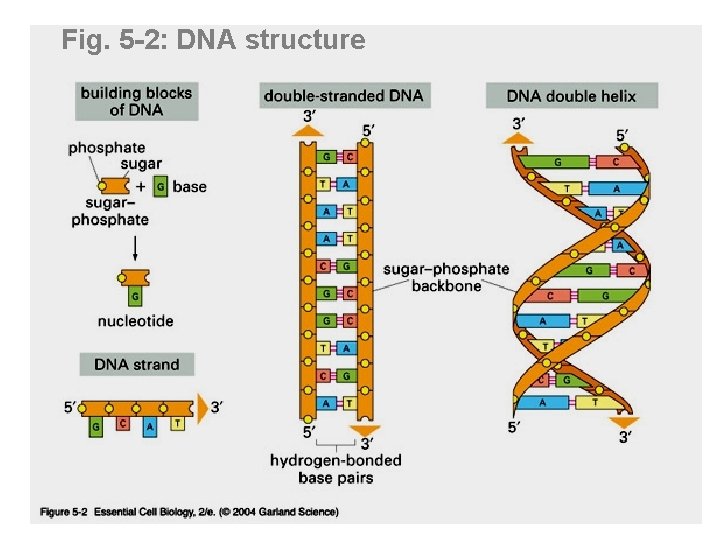
Fig. 5 -2: DNA structure

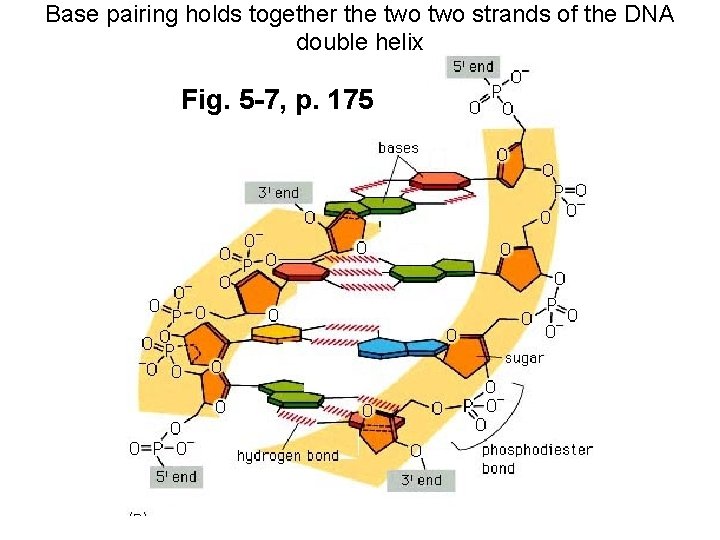
Base pairing holds together the two strands of the DNA double helix Fig. 5 -7, p. 175
![DNA is synthesized in a 5 to 3 direction Fig 6 10 p 202 DNA is synthesized in a 5’ to 3’ direction [Fig. 6 -10 (p. 202)]](https://slidetodoc.com/presentation_image_h/f9b25fa94101b216ab47b66dda1e92b4/image-46.jpg)
DNA is synthesized in a 5’ to 3’ direction [Fig. 6 -10 (p. 202)]

Watson +Crick (1962 Nobel prize) DNA model features: • DNA is composed of 2 chains forming a right-handed helix. • The chains run antiparallel to one another (5' to 3' vs. 3' to 5') • Sugar phosphate backbone (negatively charged) is hydrophilic, facing solution • Bases projecting towards the center stacked one on top of the other, form a hydrophobic core • Rules of base pairing: A pairs with T (or U in RNA); G pairs with C (Fig 5 -6 p. 175) • Purine always hydrogen bonds with pyrimidine (purines=A+G; pyrimidines=T(U)+C) • Number of hydrogen bonds varies: -The A: : T bond has two hydrogen bonds -The C: : : G bond has three hydrogen bonds
 Please pick one
Please pick one Pictionary quiz
Pictionary quiz Cubeyou
Cubeyou Please pick one
Please pick one Please pick one
Please pick one Will you please be quiet please raymond carver
Will you please be quiet please raymond carver Course title and course number
Course title and course number Course interne moyenne externe
Course interne moyenne externe How to install a lintel in a single brick wall
How to install a lintel in a single brick wall Divisibility rules lesson
Divisibility rules lesson Was ist ein handout bei einer präsentation
Was ist ein handout bei einer präsentation Basic nursing skills chapter 14
Basic nursing skills chapter 14 Dyphagia
Dyphagia Patient education handout template
Patient education handout template Journey 2050 student handout 2 word search
Journey 2050 student handout 2 word search Lecture handout
Lecture handout Bill becomes a law
Bill becomes a law Which nutrient practice was best journey 2050
Which nutrient practice was best journey 2050 Handout 5-2 graphic organizer the brain answers
Handout 5-2 graphic organizer the brain answers Wise mind handout
Wise mind handout Compassion fatigue handout for teachers
Compassion fatigue handout for teachers Karakteristik handout
Karakteristik handout Chapter 7:10 respitory system
Chapter 7:10 respitory system Orientasi
Orientasi Johari window handout
Johari window handout Wound healing nutrition handout
Wound healing nutrition handout Ciri-ciri handout
Ciri-ciri handout Cheo constipation handout
Cheo constipation handout Ciri-ciri handout
Ciri-ciri handout Odysseus travels
Odysseus travels Handout is
Handout is Py4e database handout
Py4e database handout Contoh handout pai
Contoh handout pai Rolling with resistance means
Rolling with resistance means O in fat tom
O in fat tom Hip hop referat
Hip hop referat False authority meme
False authority meme Assertive rights handout
Assertive rights handout Handouts gestalten
Handouts gestalten Handout 15-2 classical conditioning concept web
Handout 15-2 classical conditioning concept web The choice point
The choice point Handout
Handout Scatter plots and data student handout 4
Scatter plots and data student handout 4 Lee silverman voice technique
Lee silverman voice technique Spinal precautions blt
Spinal precautions blt Emotion coaching scripts
Emotion coaching scripts Fire extinguisher training handout
Fire extinguisher training handout Kpqr lrtv
Kpqr lrtv