Handling of protocol deviations PDs Cecilie Moe Head












- Slides: 22

Handling of protocol deviations (PDs) • Cecilie Moe, Head of Section, Data management • Martha Colban, Special adviser, GCP and QA

Agenda • Some general information • An SOP adapted to us? • Issues around the Protocol Deviation Handling Plan (PDHP) • An example of an electronic system for PD reporting

References

Why should you have an overview over PDs • It is a GCP requirement Meaning it is a necessary process to minimize the risk for compromising the patient safety and the data • Useful for the Data Monitoring Committees (DMCs) • It is a Clinical Study Report ICH E 3 requirement • The Regulation will require notification of Serious Breaches

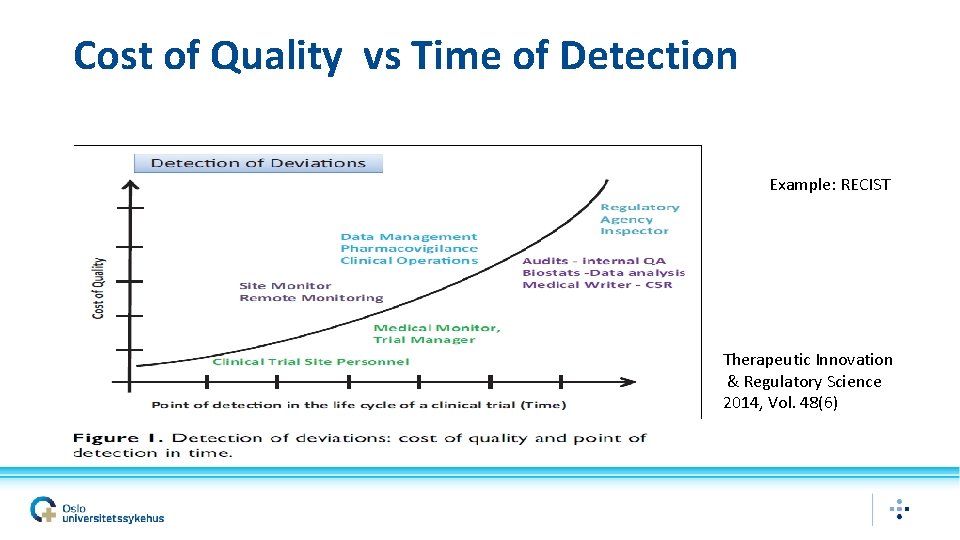
Cost of Quality vs Time of Detection Example: RECIST Therapeutic Innovation & Regulatory Science 2014, Vol. 48(6)

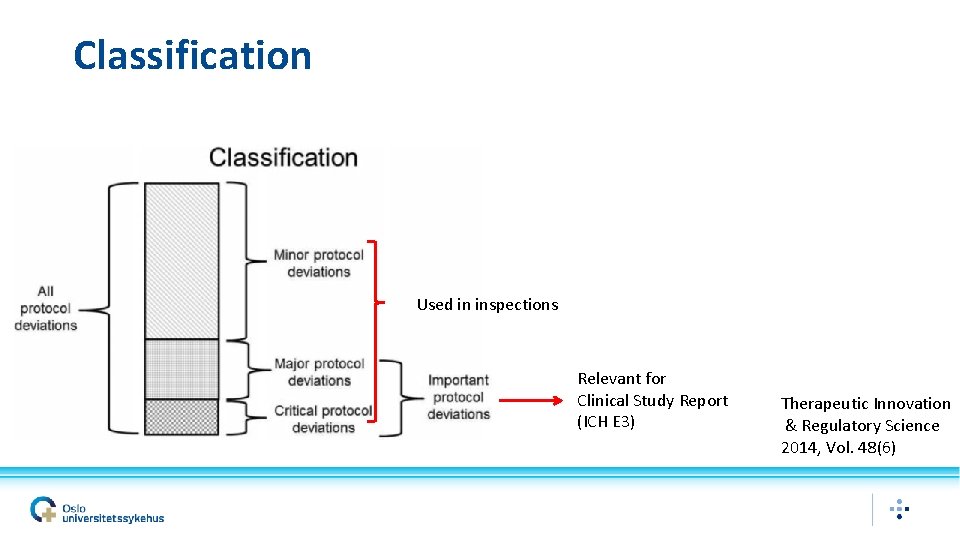
Classification Used in inspections Relevant for Clinical Study Report (ICH E 3) Therapeutic Innovation & Regulatory Science 2014, Vol. 48(6)

Organisation at Oslo University Hospital • Responsibility for the research is placed at the Department level • Most tasks are delegated to the researcher • The researcher can further delegate tasks to the CTU or others • It is not mandatory to use the CTU • OUH is an university hospital education is important

Proposed path (ref articles) • The sponsor should have an SOP for handling of PDs • There should be a PD handling plan (PDHP) for each study. Should be consistent with the Statistical Analysis Plan (SAP) • Keep in mind that the articles were published before ICH GCP R 2 was finalised and that it focuses on site deviations

The proposed SOP adapted to us

The SOP adapted to us? 3. Responsibilities The Sponsor has the overall responsibility for handling PDs. The Sponsor or their delegates are responsible for addressing protocol deviations, developing and implementing appropriate CAPAs, as well as defining the impact of deviations on analyses for inclusion in the clinical study report (CSR). PIs and study site team (eg, study coordinators) and sponsor functions are responsible for detecting protocol deviations and notifying the Sponsor as needed and implementing CAPAs. The Sponsor/CRO is responsible for assisting the site with detection and handling deviations.

References • Protocol Deviation Notification and Tracking Form (see Section 7). • Mehra M, Kurpanek K, Petrizzo M, et al. The life cycle and management of protocol deviations. Therapeutic Innovation and Regulatory Science 2014; 48(6): 762 -777. • US Food and Drug Administration. CPGM 7348. 810: Sponsors, Contract Reserach Organizations and Monitors: 2011. • US Food and Drug Administration. 21 CFR 312. 53 (c), (vi), (a) Responsibilities of Sponsors and Investigators. • Guideline for Good Clinical Practice E 6(R 1). R 2 • Structure and Content of Clinical Study Reports E 3. • Structure and Content of Clinical Study Reports Questions & Answers 21 • CFR 812. 150 (a)(4) Investigational Device Exemptions; Deviations from the investigational plan. • Guideline for the notification of serious breaches of 3 Regulation (EU) No 536/2014 or the clinical trial protocol • ISO 14155: 2011. Clinical investigation of medical devices for human subjects – Good clinical practice. Will probably be removed Will be added Not sure

Definitions • PD: Any change, divergence, or departure from the study design or procedures defined in the protocol. • Important PD: A PD that may significantly impact on the study data or the subjects For example, enrolling patients that do not meet key eligibility criteria; incorrect administration of study drug; absence of source documents; failure in recording or incorrectly recording the primary efficacy variable(s) • Not Important PD: A PD that is unlikely to have a significant effect on the study data or the subjects For example, isolated occurrence of out-of-window visit for a non-pivotal measurement.

Before study start • Ensure input to the protocol from biostatistician, research support personnel, PIs, monitors, data manager, and study site personnel, • A PDHP should be developed prior to first patient enrolled • A PD Notification and Tracking Form may be provided (note to file) or an electronic equivalent. (Will be shown later. )

During the study: Detecting, documenting and reporting PDs • What/who? Important PDs, being: 1. 2. 3. 4. 5. 6. 7. Subjects entered into the study even though they did not satisfy the entry criteria. Subjects who developed withdrawal criteria during the study but were not withdrawn. Subjects who received the wrong treatment or incorrect dose. Subjects who received an excluded concomitant treatment. Failing to collect data necessary to interpret primary endpoints Those leading to Serious Breaches (regulation) When risk identified in the risk evaluation as not acceptable have occurred and are important These PDs should be entered in the PD database on a continuous basis. Other PDs can be summarised from the database at a given frequency without tracking in the PD database.

During the study: Detecting, documenting and reporting PDs • How: Preferably a web-based system for a realtime reporting • Review: PDs detected by the sponsor will be acknowledged by PI as applicable • The Monitor/other ensures the site documents PDs according to PDHP and follows up on CAPA if applicable.

Follow-up of PDs • Review of PDs including CAPAs. • Important PDs: ongoing basis. Listings to be reviewed with other PDs • Other PDs: Along with risk evaluation, annual safety reporting, central monitoring/data validation? • Monitor/other ensures CAPA within an appropriate timeframe • Monitor/other ensures appropriate PDs have been reported to authorities if applicable • Sponsor should educate all sites about observed PDs (Newsletter? )

Non-compliance escalation • Must be defined Records • To be included in the TMF/ISF and Clinical Study Report as appropriate

Issues around the Protocol Deviation Handling Plan

The PD handling plan (PDHP) • Should be drafted at the time of protocol development • Should be a living document • Reviewed yearly? • A bit like the risk evaluation • Should it be a stand alone document or included elsewhere?

CAPA program • Risk evaluation including • • • A well written protocol Realistic windows for visits and study procedures; Limit protocol assessments to those essential to support the study endpoints; Careful consideration of prohibited medications; Development of inclusion/exclusion criteria that target the appropriate patient population; • On-going training on detection and reporting of protocol deviations (internal and/or external)

CAPA program • Discription on how PDs will be handled • • • Immediate Action (Containment) Root Cause Analysis Corrective Actions Preventive Actions Verification of Effectiveness of Corrective Action

Cathegorisation and classification • • • a) Inclusion/Exclusion Criteria b) Informed Consent c) Concomitant Medication d) Subject Visit Schedule e) Study Procedures/Assessments f) Treatment Administration g) SAE Notification/Safety Procedure h) Privacy and Data Protection i) Other? Important Deviation Not Important Deviation