Hammett plots in the world of enzymes Organic


- Slides: 8

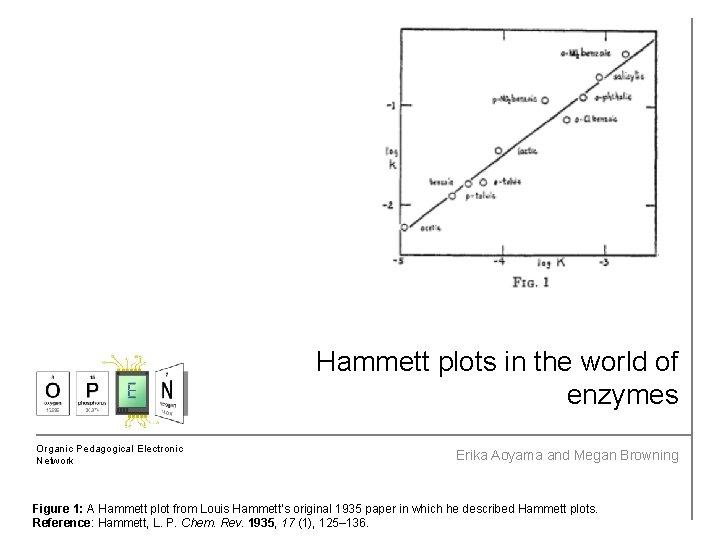
Hammett plots in the world of enzymes Organic Pedagogical Electronic Network Erika Aoyama and Megan Browning Figure 1: A Hammett plot from Louis Hammett’s original 1935 paper in which he described Hammett plots. Reference: Hammett, L. P. Chem. Rev. 1935, 17 (1), 125– 136.

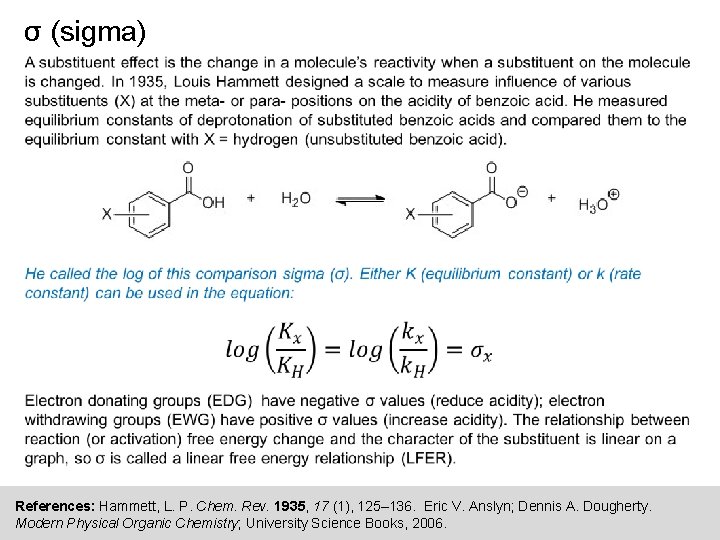
σ (sigma) References: Hammett, L. P. Chem. Rev. 1935, 17 (1), 125– 136. Eric V. Anslyn; Dennis A. Dougherty. Modern Physical Organic Chemistry; University Science Books, 2006.

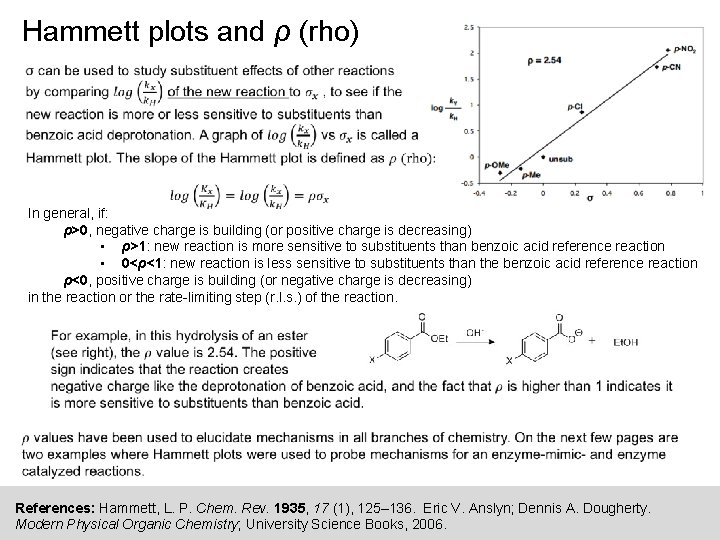
Hammett plots and ρ (rho) In general, if: ρ>0, negative charge is building (or positive charge is decreasing) • ρ>1: new reaction is more sensitive to substituents than benzoic acid reference reaction • 0<ρ<1: new reaction is less sensitive to substituents than the benzoic acid reference reaction ρ<0, positive charge is building (or negative charge is decreasing) in the reaction or the rate-limiting step (r. l. s. ) of the reaction. References: Hammett, L. P. Chem. Rev. 1935, 17 (1), 125– 136. Eric V. Anslyn; Dennis A. Dougherty. Modern Physical Organic Chemistry; University Science Books, 2006.

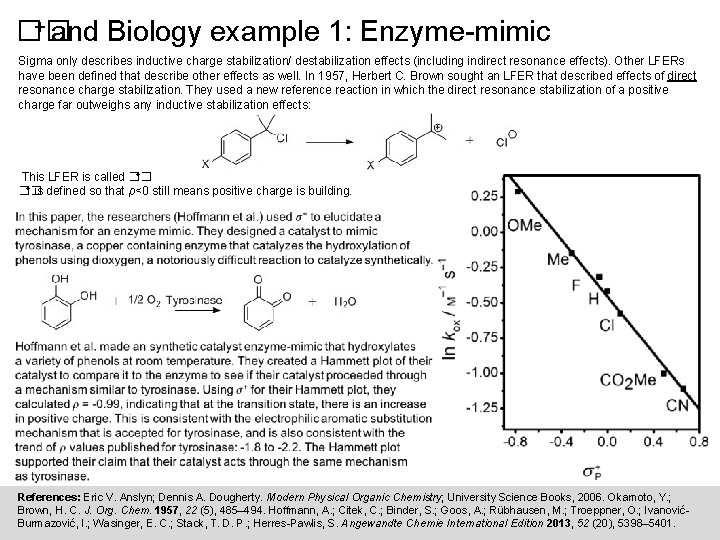
+ and Biology example 1: Enzyme-mimic �� Sigma only describes inductive charge stabilization/ destabilization effects (including indirect resonance effects). Other LFERs have been defined that describe other effects as well. In 1957, Herbert C. Brown sought an LFER that described effects of direct resonance charge stabilization. They used a new reference reaction in which the direct resonance stabilization of a positive charge far outweighs any inductive stabilization effects: +. This LFER is called �� + �� is defined so that ρ<0 still means positive charge is building. References: Eric V. Anslyn; Dennis A. Dougherty. Modern Physical Organic Chemistry; University Science Books, 2006. Okamoto, Y. ; Brown, H. C. J. Org. Chem. 1957, 22 (5), 485– 494. Hoffmann, A. ; Citek, C. ; Binder, S. ; Goos, A. ; Rübhausen, M. ; Troeppner, O. ; IvanovićBurmazović, I. ; Wasinger, E. C. ; Stack, T. D. P. ; Herres-Pawlis, S. Angewandte Chemie International Edition 2013, 52 (20), 5398– 5401.

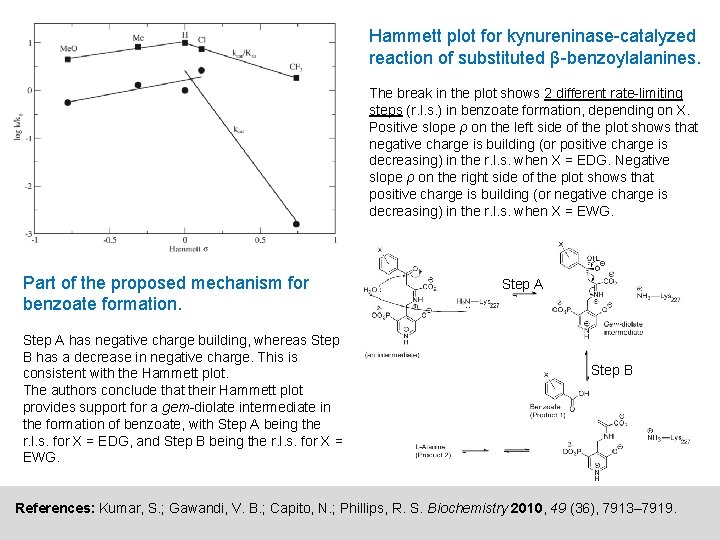
Biology example 2: Kynureninase-catalyzed reaction of β-benzoylalanine Kynureninase, an enzyme found in bacteria, catalyzes the following reaction, which is an important step in L-tryptophan catabolism: The rate-limiting part of the process is the release of the product L-Alanine. Kynureninase can catalyze a similar reaction involving β-benzoylalanine instead of L-kynurenine as the substrate: However, in this new reaction, the formation of the first product (benzoate) was found to be rate-limiting. To probe the mechanism of this new reaction, the authors synthesized derivatives of β-benzoylalanine with various substitutents (X) attached to the aromatic ring, used these derivatives as substrates for the kynureninase reaction, and created a Hammett plot from the rate data obtained (plot shown on next page). References: Kumar, S. ; Gawandi, V. B. ; Capito, N. ; Phillips, R. S. Biochemistry 2010, 49 (36), 7913– 7919.

Hammett plot for kynureninase-catalyzed reaction of substituted β-benzoylalanines. The break in the plot shows 2 different rate-limiting steps (r. l. s. ) in benzoate formation, depending on X. Positive slope ρ on the left side of the plot shows that negative charge is building (or positive charge is decreasing) in the r. l. s. when X = EDG. Negative slope ρ on the right side of the plot shows that positive charge is building (or negative charge is decreasing) in the r. l. s. when X = EWG. Part of the proposed mechanism for benzoate formation. Step A has negative charge building, whereas Step B has a decrease in negative charge. This is consistent with the Hammett plot. The authors conclude that their Hammett plot provides support for a gem-diolate intermediate in the formation of benzoate, with Step A being the r. l. s. for X = EDG, and Step B being the r. l. s. for X = EWG. Step A Step B References: Kumar, S. ; Gawandi, V. B. ; Capito, N. ; Phillips, R. S. Biochemistry 2010, 49 (36), 7913– 7919.

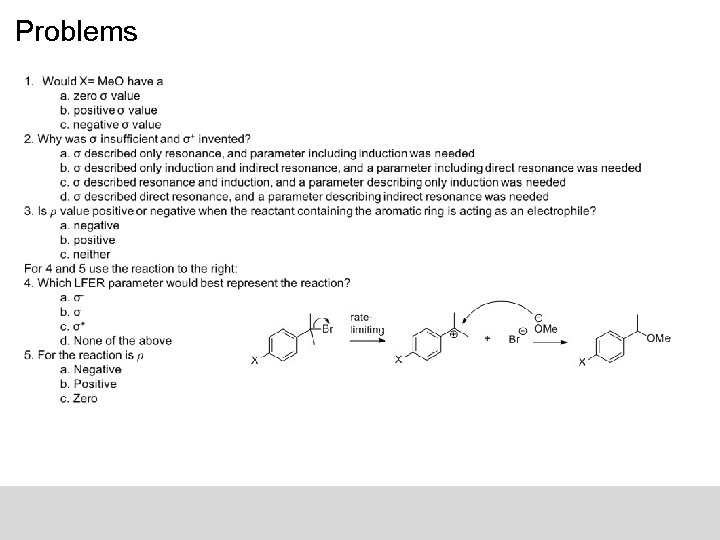
Problems

Contributed by: Erika Aoyama and Megan Browning University of Utah, 2016 This work is licensed under a Creative Commons Attribution. Share. Alike 4. 0 International License.