Halogenoalkanes and Reaction Pathways IB Chemistry Halogenoalkanes Halogenoalkanes
Halogenoalkanes and Reaction Pathways IB Chemistry

Halogenoalkanes

Halogenoalkanes l l l Halogenoalkanes consist of a carbon bonded to an atom of fluorine, chlorine or bromine General formula = Cn. H 2 n+1 X, where X is a halogen These are typically oily liquids that do not mix well with water They are used in many products CFCs have been renowned for their negative impact on the ozone layer
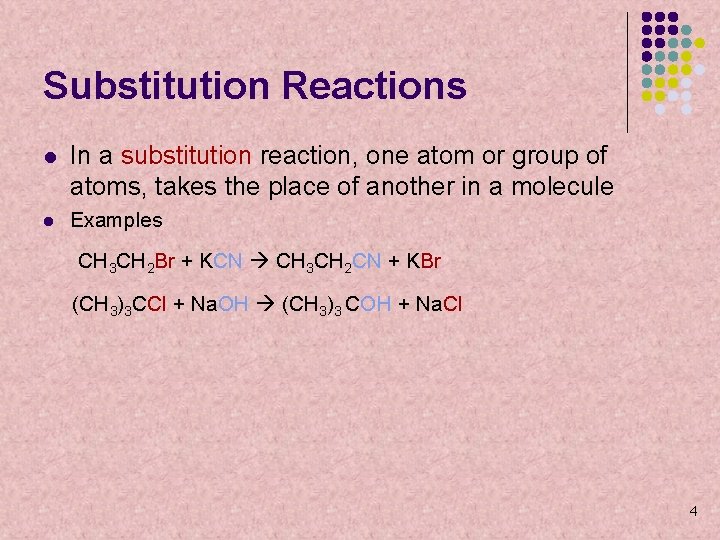
Substitution Reactions l In a substitution reaction, one atom or group of atoms, takes the place of another in a molecule l Examples CH 3 CH 2 Br + KCN CH 3 CH 2 CN + KBr (CH 3)3 CCl + Na. OH (CH 3)3 COH + Na. Cl 4

Nucleophilic Substitution l l l A nucleophile is a molecule or ion that has a high electron density… nucleo = nucleus; phile = loving. It is attracted to atoms in molecules with a lower electron density. It may replace another group in an organic molecule, such as a halogen. The hydroxide ion (OH-) from Na. OH is an effective nucleophile that will substitute the halogen, turning the product into an alcohol These reactions are known as substitution nucleophilic, or SN reactions. 5
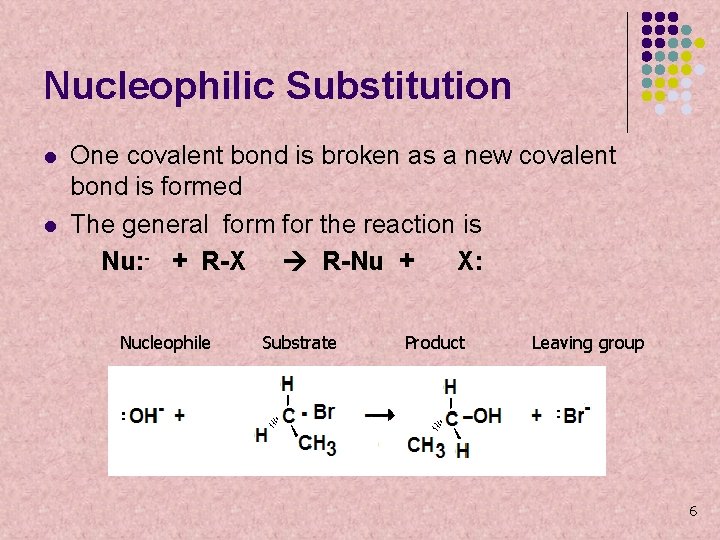
Nucleophilic Substitution l l One covalent bond is broken as a new covalent bond is formed The general form for the reaction is Nu: - + R-X R-Nu + X: Nucleophile Substrate Product Leaving group 6
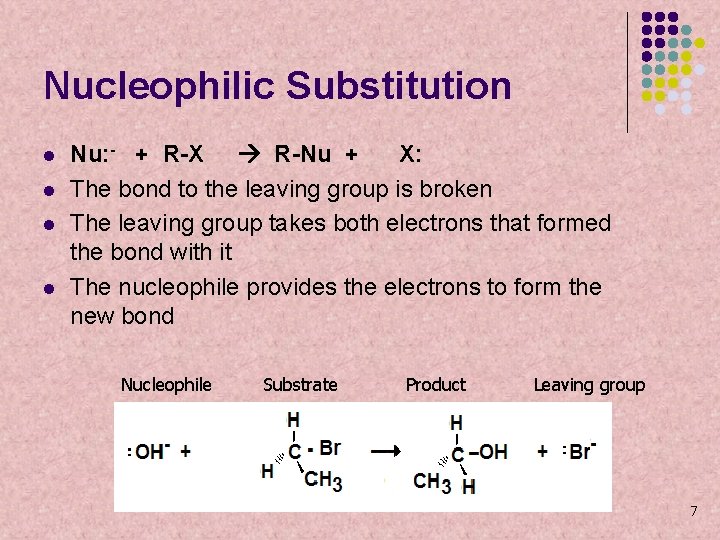
Nucleophilic Substitution l l Nu: - + R-X R-Nu + X: The bond to the leaving group is broken The leaving group takes both electrons that formed the bond with it The nucleophile provides the electrons to form the new bond Nucleophile Substrate Product Leaving group 7
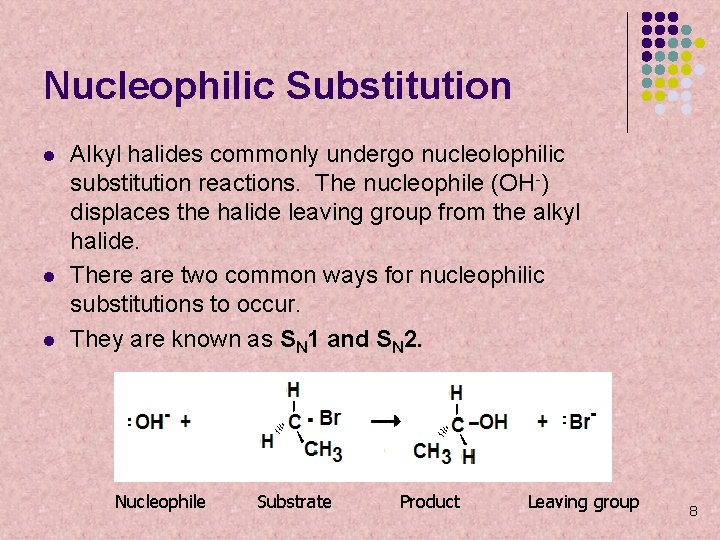
Nucleophilic Substitution l l l Alkyl halides commonly undergo nucleolophilic substitution reactions. The nucleophile (OH-) displaces the halide leaving group from the alkyl halide. There are two common ways for nucleophilic substitutions to occur. They are known as SN 1 and SN 2. Nucleophile Substrate Product Leaving group 8
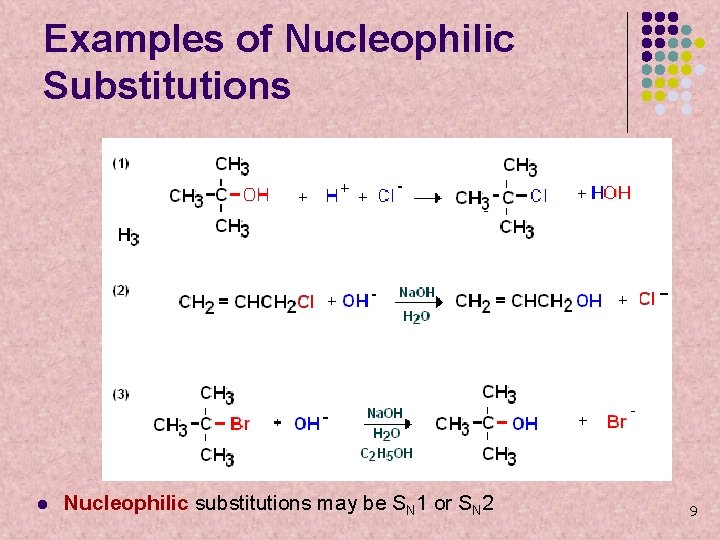
Examples of Nucleophilic Substitutions l Nucleophilic substitutions may be SN 1 or SN 2 9
Nucleophilic Substitution Bimolecular or SN 2 l l l A reaction is bimolecular when the rate depends on both the concentration of 2 reactants: the substrate and the nucleophile. SN 2 mechanisms occur most readily with methyl compounds and primary haloalkanes Takes place in one step 10
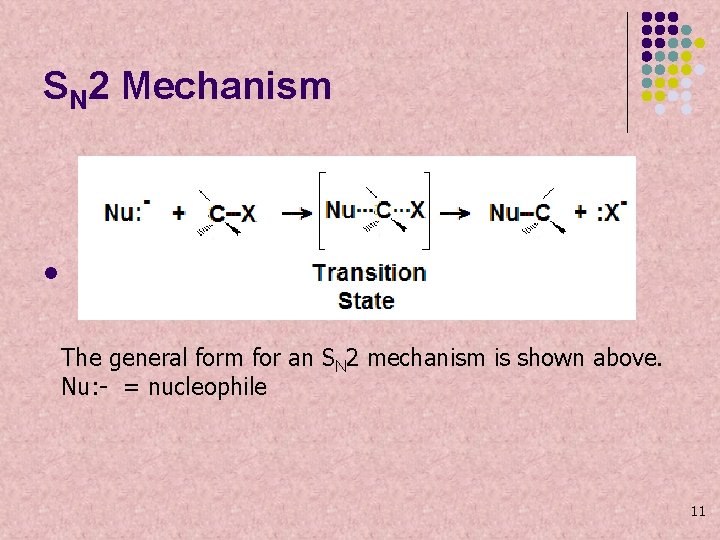
SN 2 Mechanism l The general form for an SN 2 mechanism is shown above. Nu: - = nucleophile 11
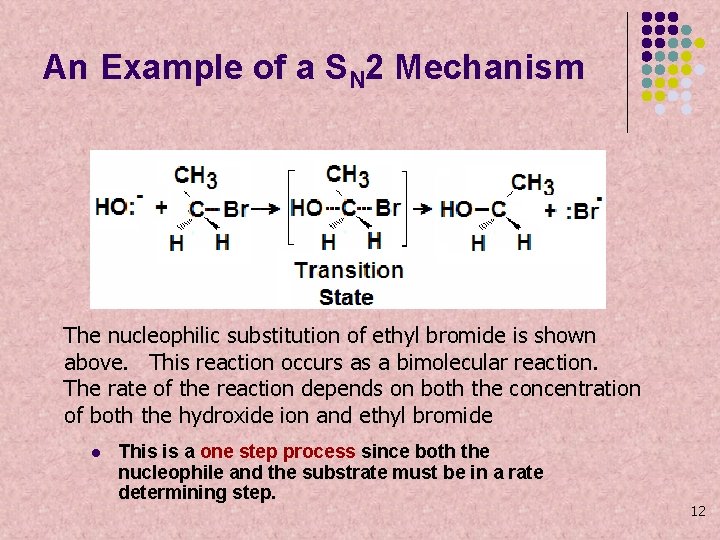
An Example of a SN 2 Mechanism The nucleophilic substitution of ethyl bromide is shown above. This reaction occurs as a bimolecular reaction. The rate of the reaction depends on both the concentration of both the hydroxide ion and ethyl bromide l This is a one step process since both the nucleophile and the substrate must be in a rate determining step. 12
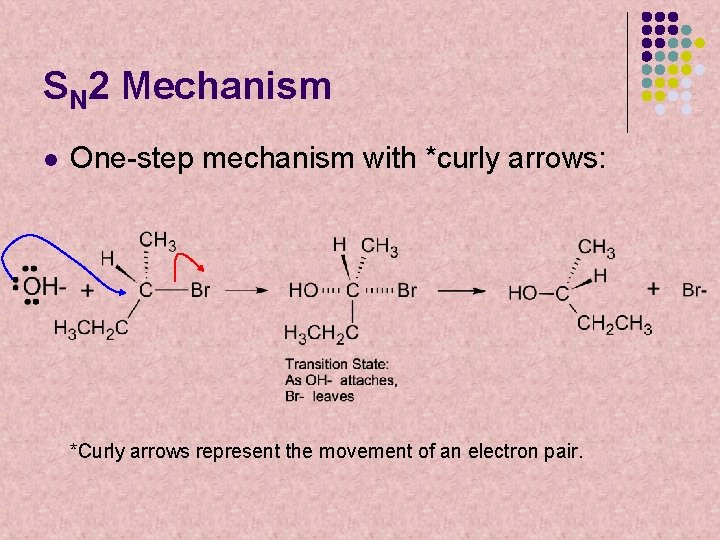
SN 2 Mechanism l One-step mechanism with *curly arrows: *Curly arrows represent the movement of an electron pair.
Nucleophilic Substitution Unimolecular or SN 1 l l l A unimolecular reaction occurs when the rate of reaction depends on the concentration of 1 reactant: the substrate but not the nucleophile. A unimolecular reaction is a two step process since the subtrate and the nucleophile cannot both appear in the rate determining step SN 1 mechanisms occur most readily with tertiary haloalkanes and some secondary haloalkanes. 14
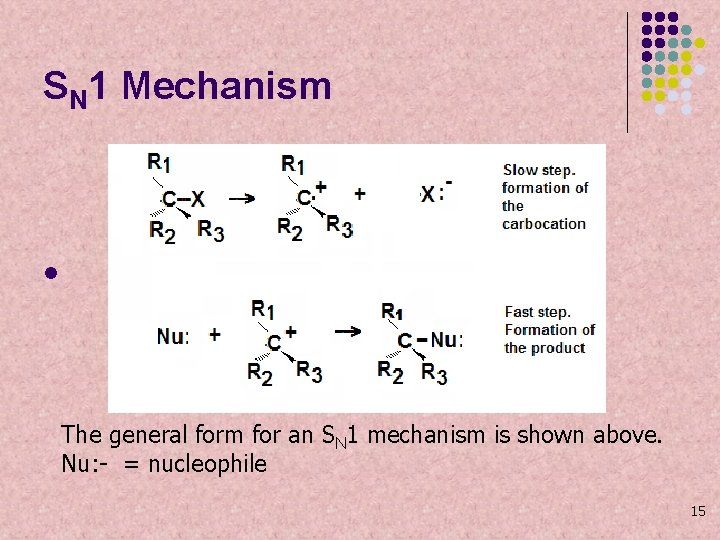
SN 1 Mechanism l The general form for an SN 1 mechanism is shown above. Nu: - = nucleophile 15
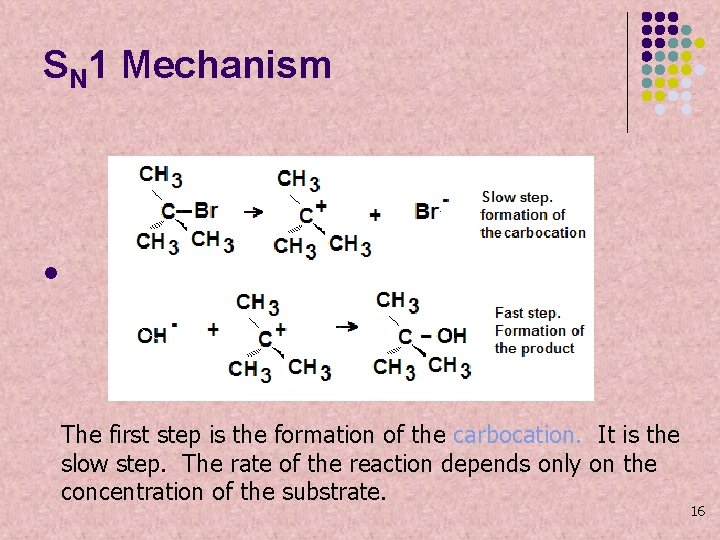
SN 1 Mechanism l The first step is the formation of the carbocation. It is the slow step. The rate of the reaction depends only on the concentration of the substrate. 16
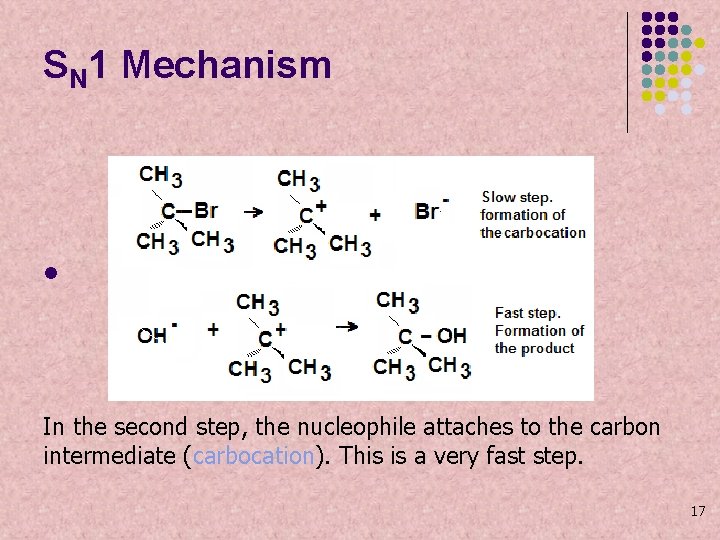
SN 1 Mechanism l In the second step, the nucleophile attaches to the carbon intermediate (carbocation). This is a very fast step. 17
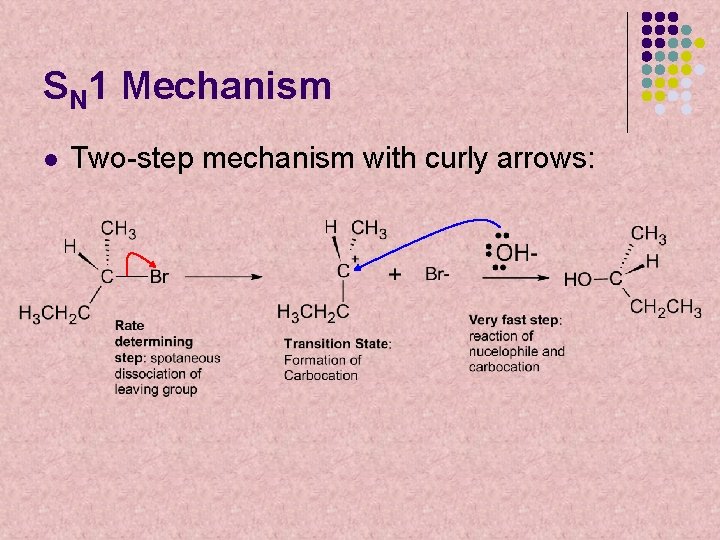
SN 1 Mechanism l Two-step mechanism with curly arrows:
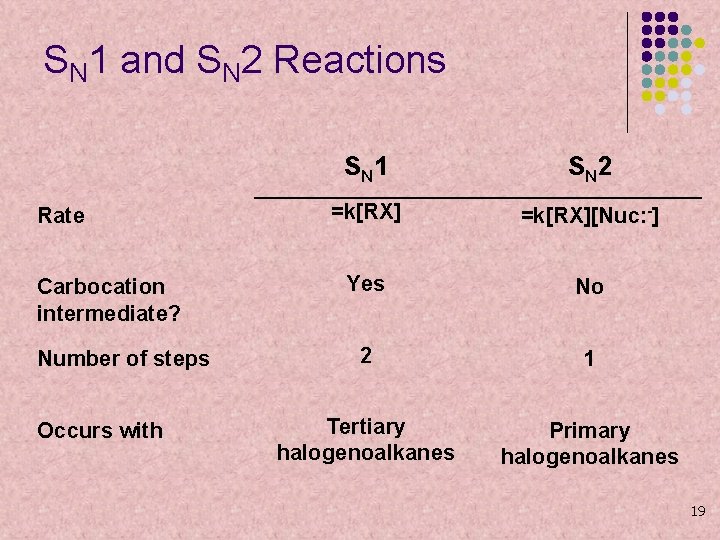
SN 1 and SN 2 Reactions Rate Carbocation intermediate? Number of steps Occurs with S N 1 S N 2 =k[RX][Nuc: -] Yes No 2 1 Tertiary halogenoalkanes Primary halogenoalkanes 19

l l http: //www. youtube. com/watch? v=t. Aj. Fra. Tw 0 HM http: //www. youtube. com/watch? v=Ztn. AR 3 u. O Abo (? ) 20
Reaction Pathways and mechanisms l l l Most organic reactions proceed by a defined sequence or set of steps. The detailed pathway which an organic reaction follows is called a mechanism. Knowing a reaction mechanism is very valuable information. It allows the chemist to predict what products will be formed when a chemical reaction occurs. The organic chemist can use this information to modify compounds and to synthesize new compounds with certain desired characteristics. 21
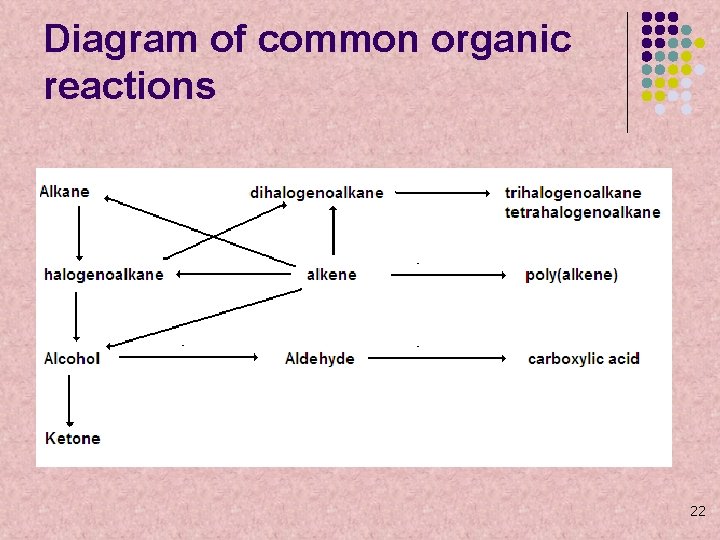
Diagram of common organic reactions 22

Reaction Pathway Practice l l Fill in the reaction pathway chart, showing the necessary reactants and any other additional conditions necessary for the reaction to take place You may omit trihalogenoalkanes and poly(alkenes)
- Slides: 23