Halogen Purpose 1 Verify the physical and chemical


- Slides: 6

Halogen ★ Purpose: 1. Verify the physical and chemical properties of halogen, haloid oxyacid, haloid oxysalt and hydrogen halide. 2. Master the methods of how to prepare halogen. 3. Learn how to identify the halogen ions.

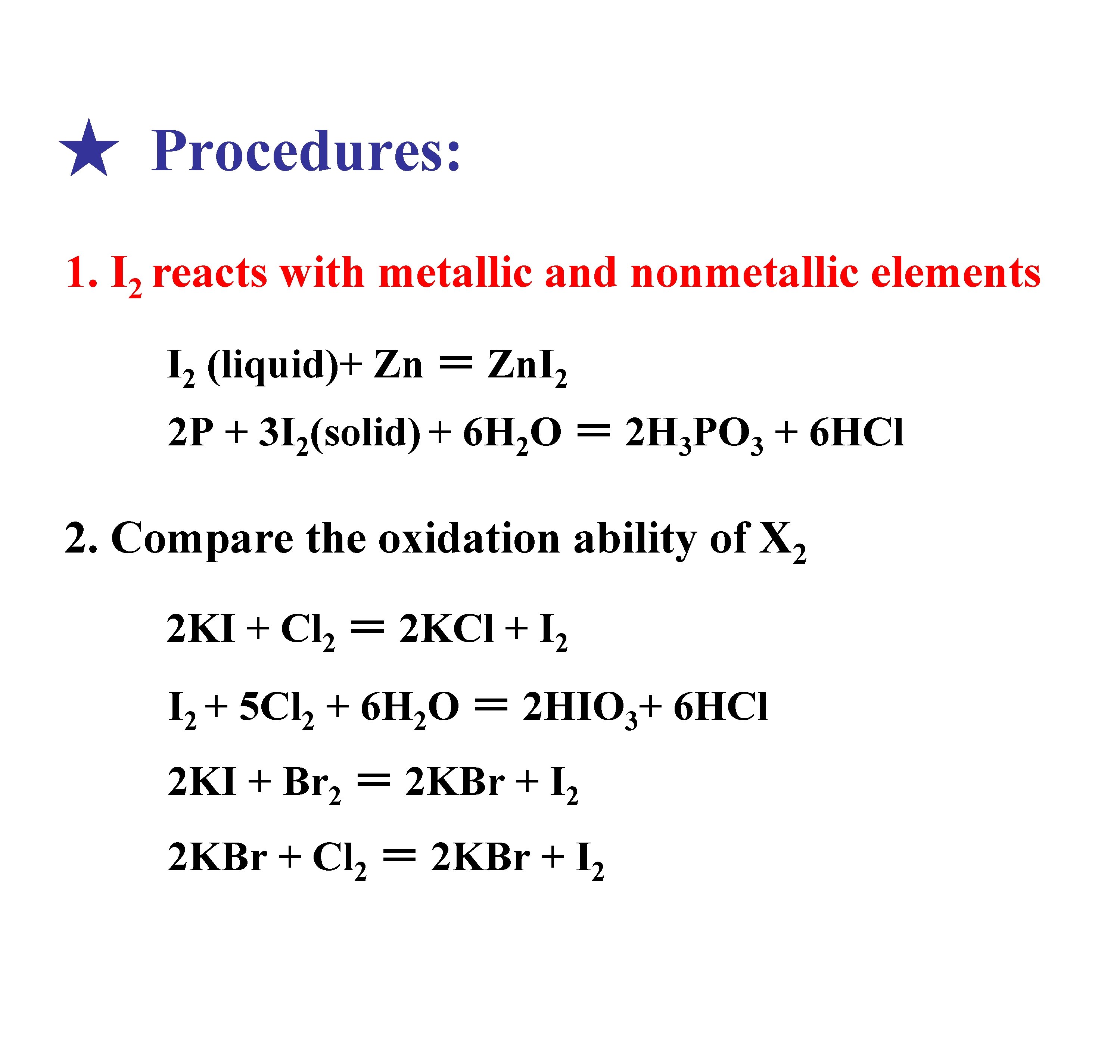
★ Procedures: 1. I 2 reacts with metallic and nonmetallic elements I 2 (liquid)+ Zn = Zn. I 2 2 P + 3 I 2(solid) + 6 H 2 O = 2 H 3 PO 3 + 6 HCl 2. Compare the oxidation ability of X 2 2 KI + Cl 2 = 2 KCl + I 2 + 5 Cl 2 + 6 H 2 O = 2 HIO 3+ 6 HCl 2 KI + Br 2 = 2 KBr + I 2 2 KBr + Cl 2 = 2 KBr + I 2

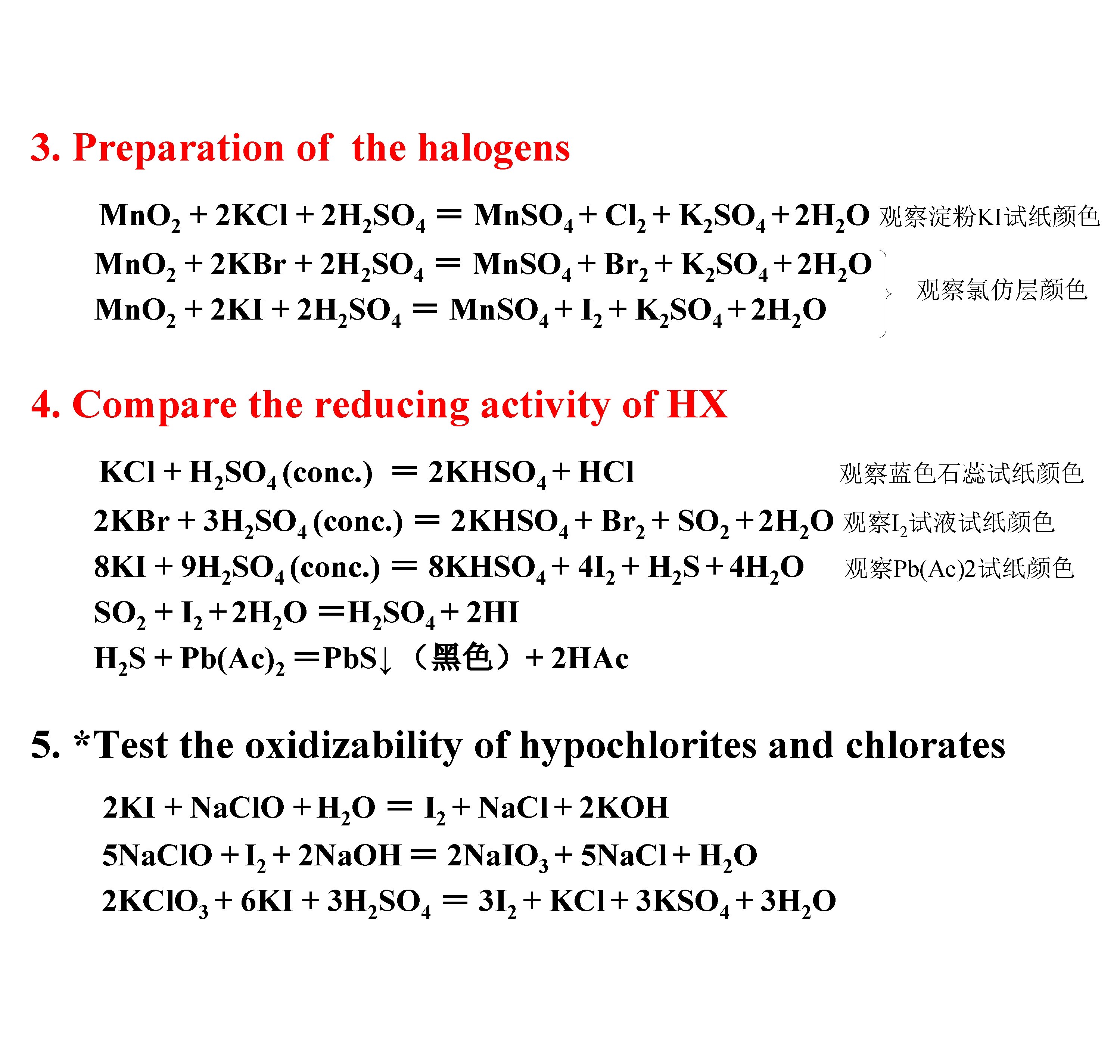
3. Preparation of the halogens Mn. O 2 + 2 KCl + 2 H 2 SO 4 = Mn. SO 4 + Cl 2 + K 2 SO 4 + 2 H 2 O 观察淀粉KI试纸颜色 Mn. O 2 + 2 KBr + 2 H 2 SO 4 = Mn. SO 4 + Br 2 + K 2 SO 4 + 2 H 2 O Mn. O 2 + 2 KI + 2 H 2 SO 4 = Mn. SO 4 + I 2 + K 2 SO 4 + 2 H 2 O 观察氯仿层颜色 4. Compare the reducing activity of HX KCl + H 2 SO 4 (conc. ) = 2 KHSO 4 + HCl 观察蓝色石蕊试纸颜色 2 KBr + 3 H 2 SO 4 (conc. ) = 2 KHSO 4 + Br 2 + SO 2 + 2 H 2 O 观察I 2试液试纸颜色 8 KI + 9 H 2 SO 4 (conc. ) = 8 KHSO 4 + 4 I 2 + H 2 S + 4 H 2 O 观察Pb(Ac)2试纸颜色 SO 2 + I 2 + 2 H 2 O =H 2 SO 4 + 2 HI H 2 S + Pb(Ac)2 =Pb. S↓ (黑色)+ 2 HAc 5. *Test the oxidizability of hypochlorites and chlorates 2 KI + Na. Cl. O + H 2 O = I 2 + Na. Cl + 2 KOH 5 Na. Cl. O + I 2 + 2 Na. OH = 2 Na. IO 3 + 5 Na. Cl + H 2 O 2 KCl. O 3 + 6 KI + 3 H 2 SO 4 = 3 I 2 + KCl + 3 KSO 4 + 3 H 2 O

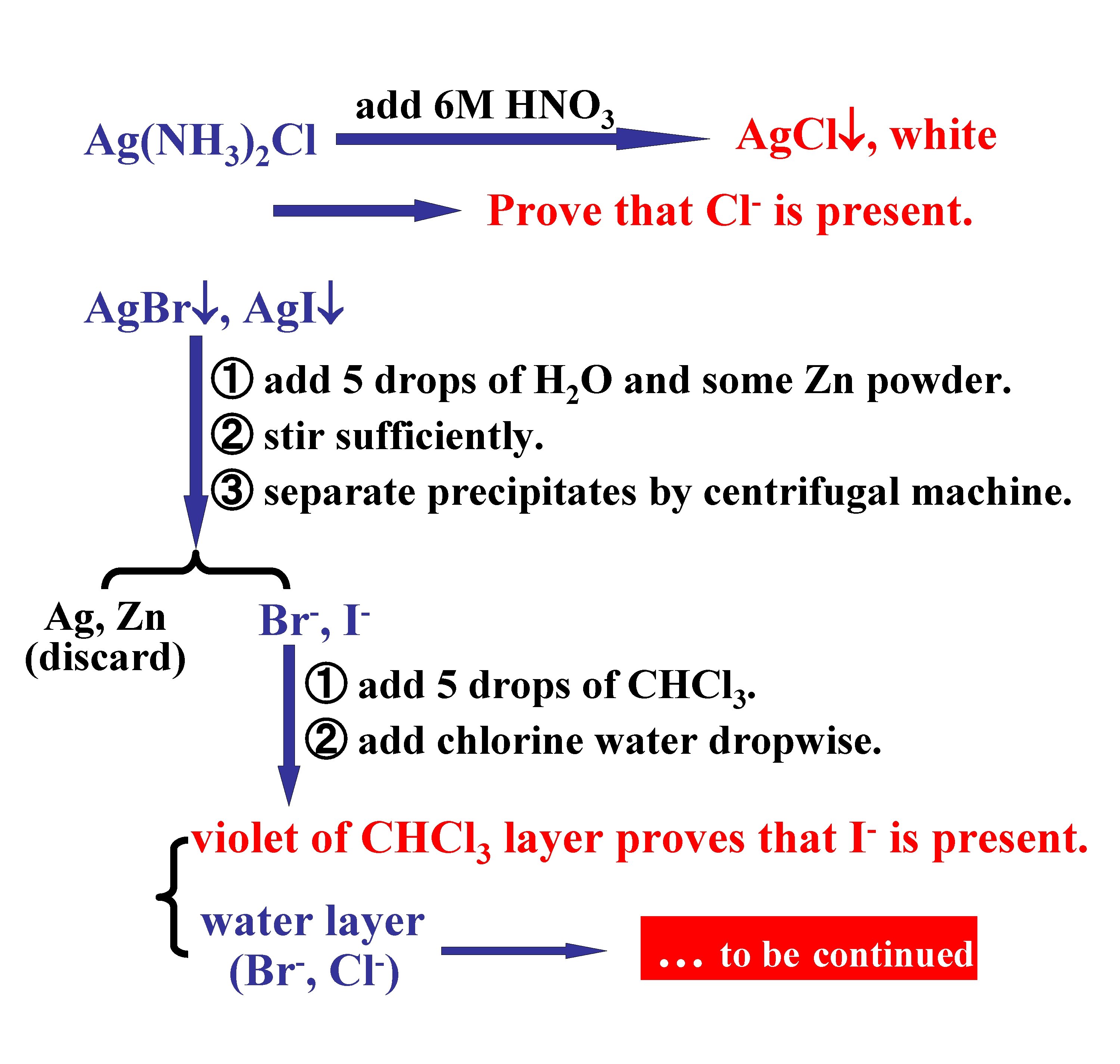
6. *Separation and detection of Cl , Br , and I. Br , I ① add 6 M HNO 3 to acidify the solution. ② add 0. 1 M Ag. NO 3 until precipitated completely. ③ separate Ag. X by centrifugal machine. Solution (discard) Ag. Cl , Ag. Br , Ag. I Ag(NH 3)2 Cl ① wash Ag. X with distilled water. ② add 12% (NH 4)2 CO 3 2 ml, stirring. ③ separate precipitates by centrifugal machine. Ag. Br , Ag. I … to be continued

Ag(NH 3)2 Cl add 6 M HNO 3 Ag. Cl , white Prove that Cl is present. Ag. Br , Ag. I ① add 5 drops of H 2 O and some Zn powder. ② stir sufficiently. ③ separate precipitates by centrifugal machine. Ag, Zn (discard) Br , I ① add 5 drops of CHCl 3. ② add chlorine water dropwise. violet of CHCl 3 layer proves that water layer (Br , Cl ) I is present. … to be continued

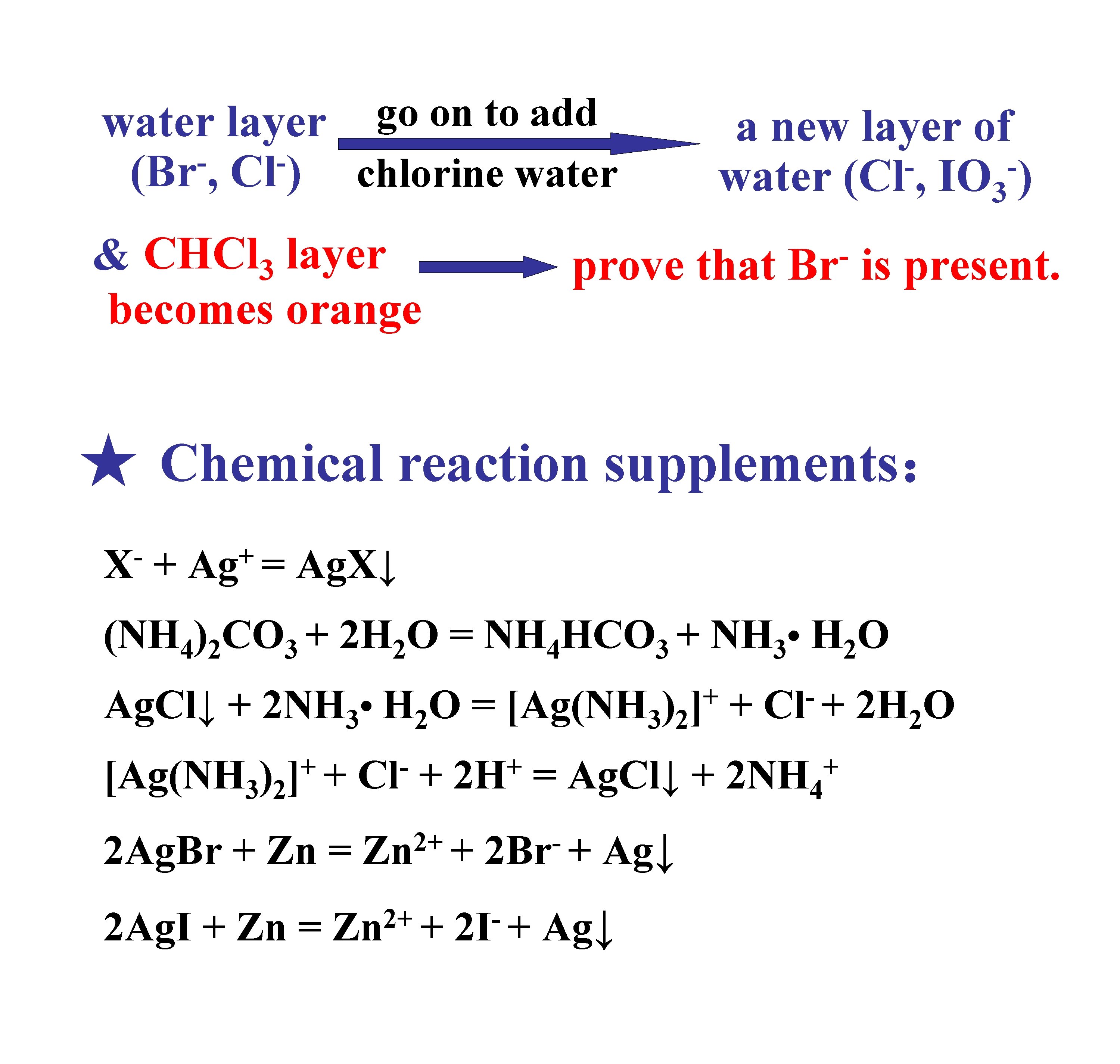
go on to add water layer (Br , Cl ) chlorine water & CHCl 3 layer becomes orange a new layer of water (Cl , IO 3 ) prove that Br is present. ★ Chemical reaction supplements: X + + Ag = Ag. X↓ (NH 4)2 CO 3 + 2 H 2 O = NH 4 HCO 3 + NH 3 • H 2 O Ag. Cl↓ + 2 NH 3 • H 2 O = + [Ag(NH 3)2] + Cl 2 Ag. Br + Zn = 2+ Zn + 2 Ag. I + Zn = + 2+ Zn + + [Ag(NH 3)2] + 2 H = Ag. Cl↓ + 2 Br + 2 I + Ag↓ + Cl + + 2 NH 4 2 H 2 O