HALIDE ION TESTS TEST FOR HALIDE IONS Cl





- Slides: 18

HALIDE ION TESTS

TEST FOR HALIDE IONS (Cl- ; Br- ; I-) ADD DILUTE NITRIC ACID FOLLOWED BY SILVER NITRATE SOLUTION TO THE SAMPLE SOLUTION NITRIC ACID IS ADDED TO REMOVE IMPURITES IT IS THE SILVER NITRATE SOLUTION THAT REACTS WITH THE SAMPLE SOLUTION A COLOURED PRECIPITATE OF SILVER HALIDES FORM


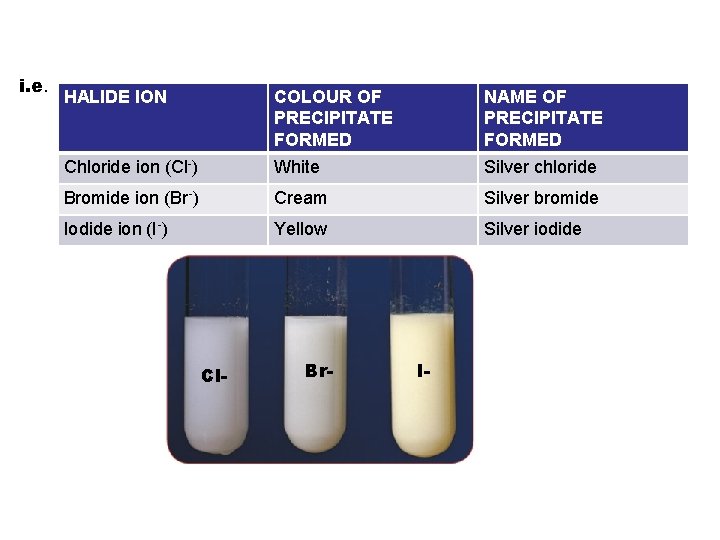
i. e. HALIDE ION Chloride ion (Cl-) COLOUR OF PRECIPITATE FORMED White NAME OF PRECIPITATE FORMED Silver chloride Bromide ion (Br-) Cream Silver bromide Iodide ion (I-) Yellow Silver iodide Cl- Br- I-

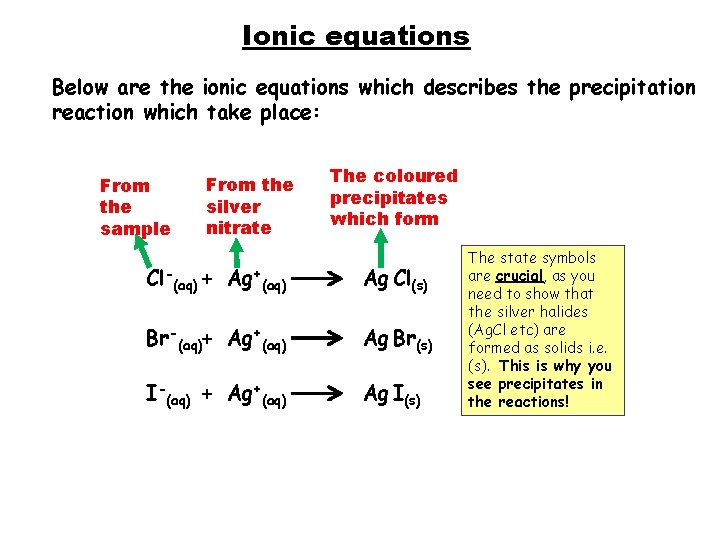
Ionic equations Below are the ionic equations which describes the precipitation reaction which take place: From the sample From the silver nitrate The coloured precipitates which form Cl-(aq) + Ag+(aq) Ag Cl(s) Br-(aq)+ Ag+(aq) Ag Br(s) I-(aq) + Ag+(aq) Ag I(s) The state symbols are crucial, as you need to show that the silver halides (Ag. Cl etc) are formed as solids i. e. (s). This is why you see precipitates in the reactions!

The main problem with this test is that it is very difficult to distinguish between the precipitate colours particularly between Ag. Br and Ag. I so another confirmatory test must be carried out To carry out a confirmatory test solution is added ammonia

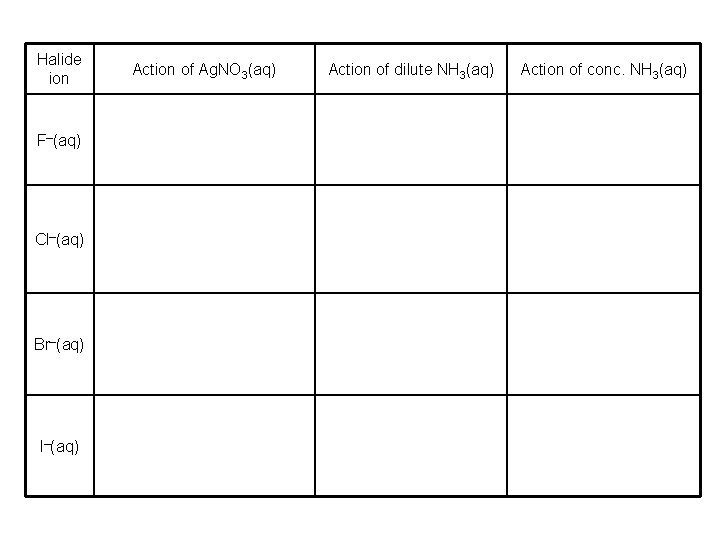
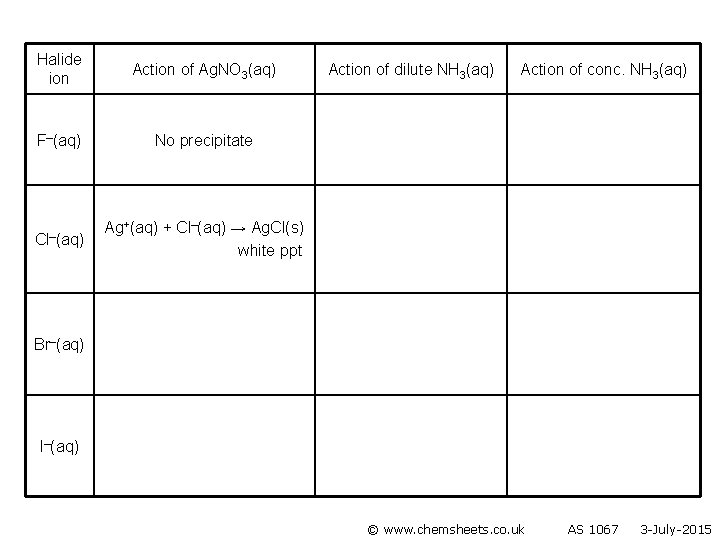
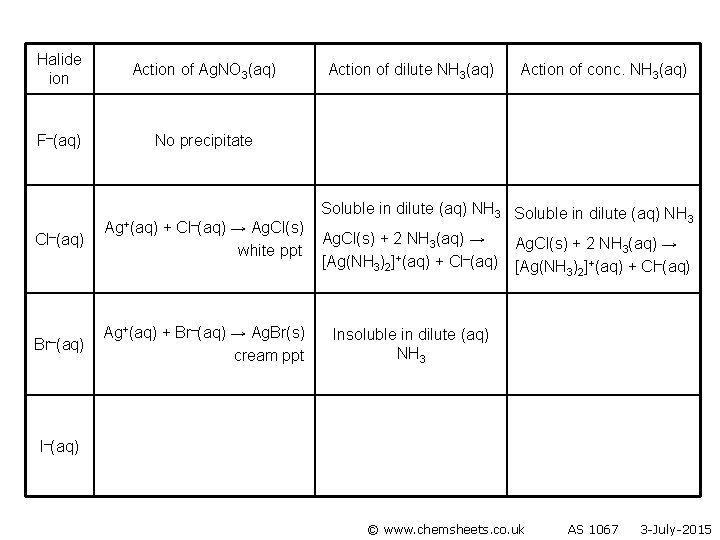
Halide ion F–(aq) Cl–(aq) Br–(aq) I–(aq) Action of Ag. NO 3(aq) Action of dilute NH 3(aq) Action of conc. NH 3(aq)

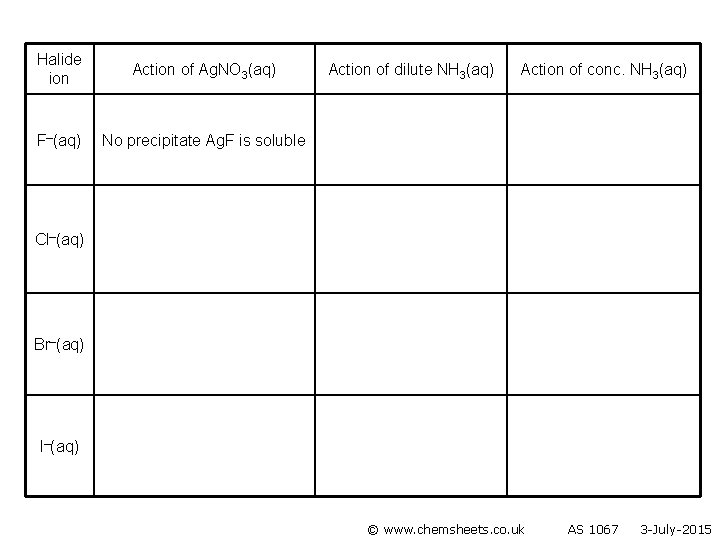
Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Ag. F is soluble Action of dilute NH 3(aq) Action of conc. NH 3(aq) Cl–(aq) Br–(aq) I–(aq) © www. chemsheets. co. uk AS 1067 3 -July-2015

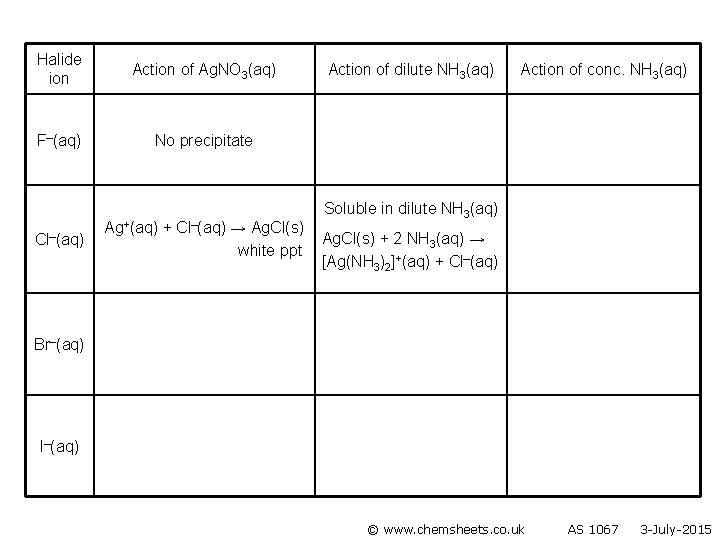
Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Action of dilute NH 3(aq) Action of conc. NH 3(aq) Br–(aq) I–(aq) © www. chemsheets. co. uk AS 1067 3 -July-2015

Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Action of dilute NH 3(aq) Action of conc. NH 3(aq) Soluble in dilute NH 3(aq) Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Br–(aq) I–(aq) © www. chemsheets. co. uk AS 1067 3 -July-2015

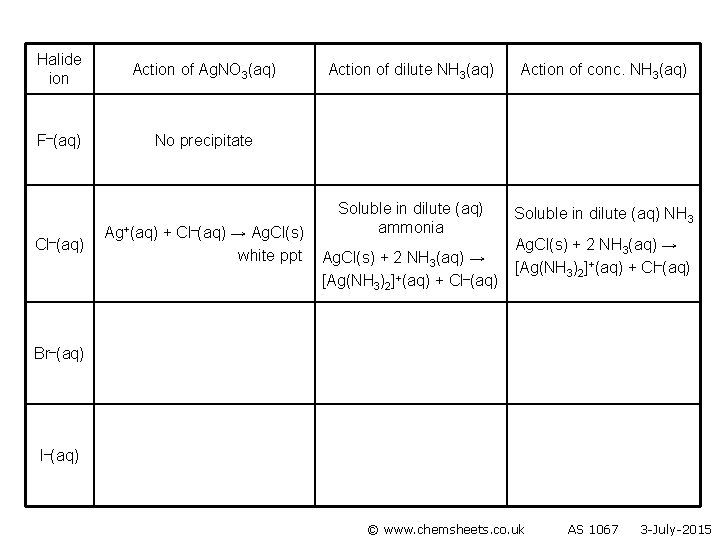
Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Action of dilute NH 3(aq) Action of conc. NH 3(aq) Soluble in dilute (aq) ammonia Soluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Br–(aq) I–(aq) © www. chemsheets. co. uk AS 1067 3 -July-2015

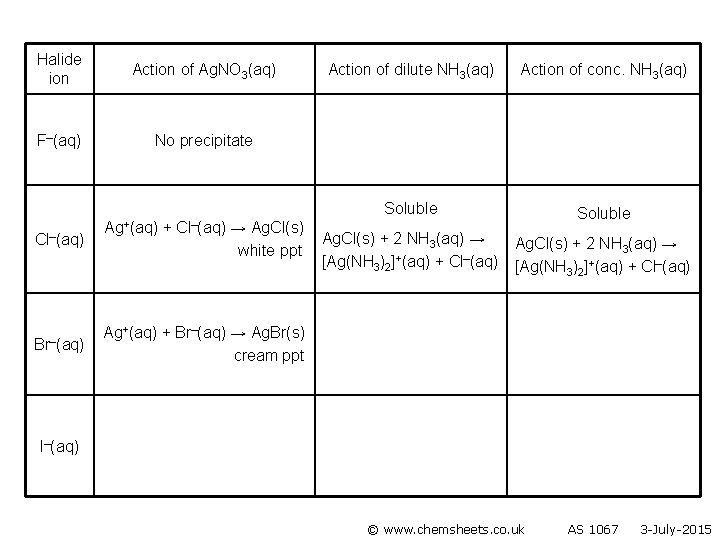
Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Br–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Action of dilute NH 3(aq) Action of conc. NH 3(aq) Soluble Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Ag+(aq) + Br–(aq) → Ag. Br(s) cream ppt I–(aq) © www. chemsheets. co. uk AS 1067 3 -July-2015

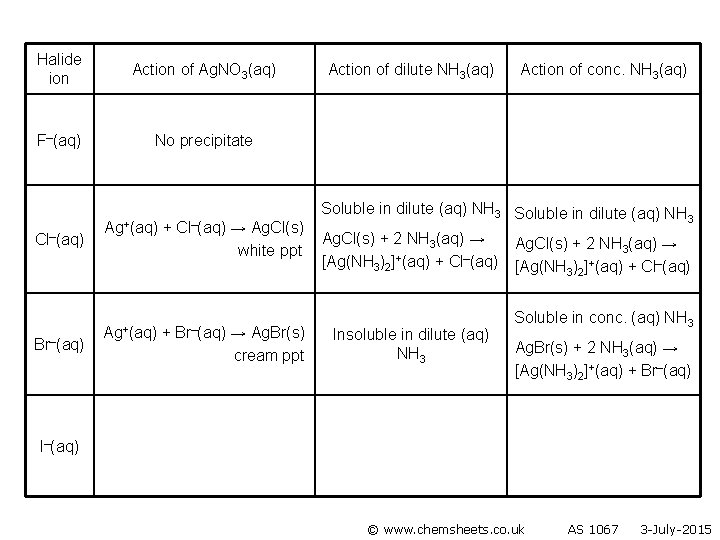
Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Br–(aq) Ag+(aq) + Br–(aq) → Ag. Br(s) cream ppt Action of dilute NH 3(aq) Action of conc. NH 3(aq) Soluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Insoluble in dilute (aq) NH 3 I–(aq) © www. chemsheets. co. uk AS 1067 3 -July-2015

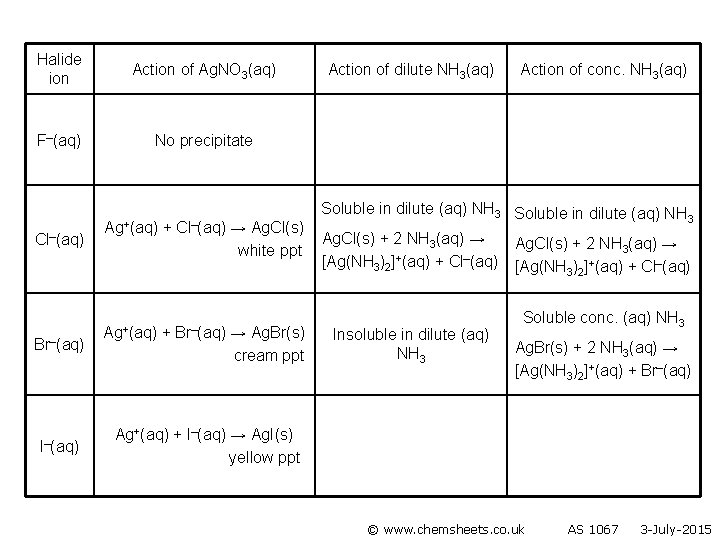
Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Br–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Ag+(aq) + Br–(aq) → Ag. Br(s) cream ppt Action of dilute NH 3(aq) Action of conc. NH 3(aq) Soluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Insoluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Soluble in conc. (aq) NH 3 Ag. Br(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Br–(aq) I–(aq) © www. chemsheets. co. uk AS 1067 3 -July-2015

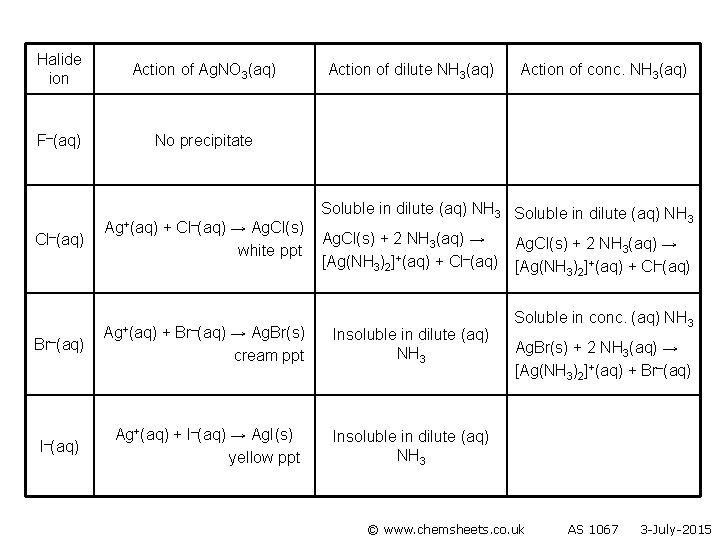
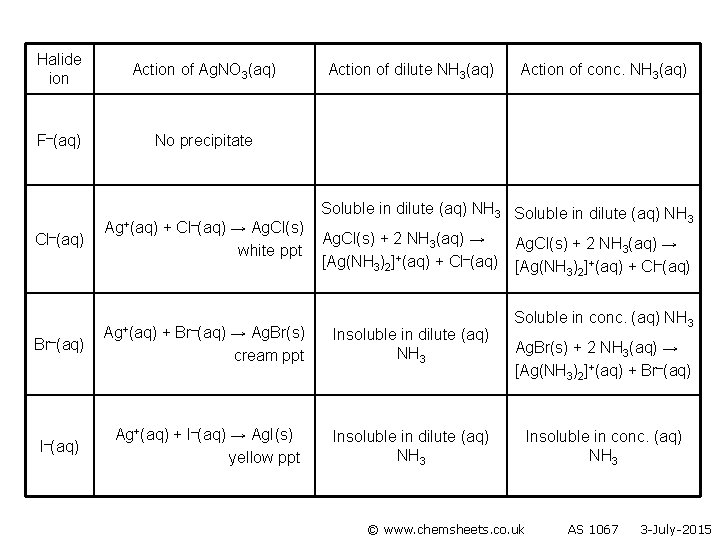
Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Br–(aq) I–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Ag+(aq) + Br–(aq) → Ag. Br(s) cream ppt Action of dilute NH 3(aq) Action of conc. NH 3(aq) Soluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Insoluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Soluble conc. (aq) NH 3 Ag. Br(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Br–(aq) Ag+(aq) + I–(aq) → Ag. I(s) yellow ppt © www. chemsheets. co. uk AS 1067 3 -July-2015

Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Br–(aq) I–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Ag+(aq) + Br–(aq) Action of dilute NH 3(aq) Action of conc. NH 3(aq) Soluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) → Ag. Br(s) cream ppt Insoluble in dilute (aq) NH 3 Ag+(aq) + I–(aq) → Ag. I(s) yellow ppt Insoluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Soluble in conc. (aq) NH 3 Ag. Br(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Br–(aq) © www. chemsheets. co. uk AS 1067 3 -July-2015

Halide ion Action of Ag. NO 3(aq) F–(aq) No precipitate Cl–(aq) Br–(aq) I–(aq) Ag+(aq) + Cl–(aq) → Ag. Cl(s) white ppt Ag+(aq) + Br–(aq) Action of dilute NH 3(aq) Action of conc. NH 3(aq) Soluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) → Ag. Br(s) cream ppt Insoluble in dilute (aq) NH 3 Ag+(aq) + I–(aq) → Ag. I(s) yellow ppt Insoluble in dilute (aq) NH 3 Ag. Cl(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Cl–(aq) Soluble in conc. (aq) NH 3 Ag. Br(s) + 2 NH 3(aq) → [Ag(NH 3)2]+(aq) + Br–(aq) © www. chemsheets. co. uk Insoluble in conc. (aq) NH 3 AS 1067 3 -July-2015
