Half Life Half Life Halflife is the time











- Slides: 28

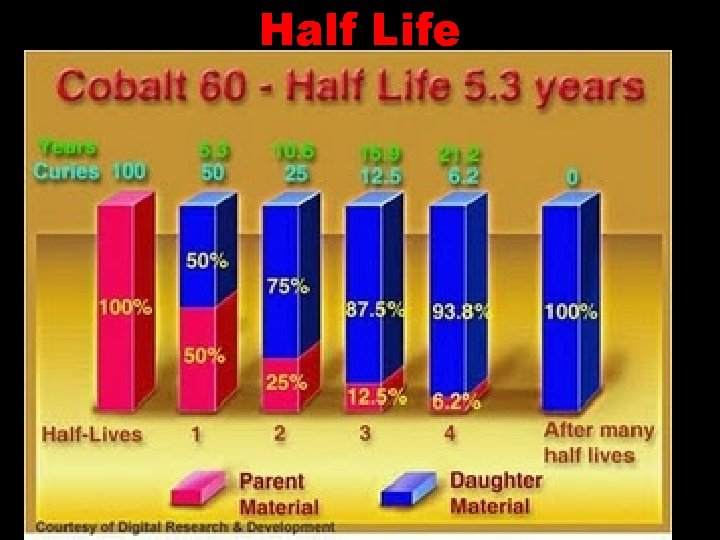
Half Life

Half Life Half-life is the time it takes for half of the atoms of a sample to decay. For example: • A student was testing a sample of 8 grams of radioactive protactinium. Protactinium has a a half life of 1 minute and decays into actinium. • After 1 minute there would be 4 g of protactinium (and 4 g of actinium). • After 2 minutes there would be 2 g of protactinium remaining (and now 6 g of actinium). • After 3 minutes there would be 1 g of protactinium remaining (and now 7 g of actinium)

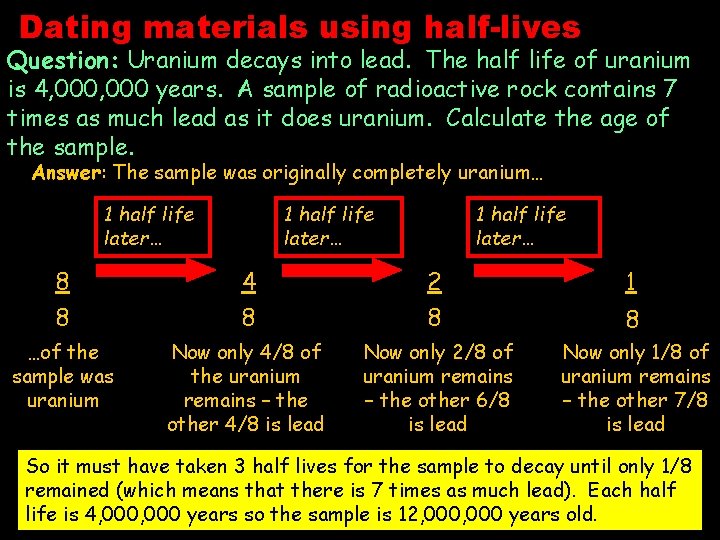
Dating materials using half-lives Question: Uranium decays into lead. The half life of uranium is 4, 000 years. A sample of radioactive rock contains 7 times as much lead as it does uranium. Calculate the age of the sample. Answer: The sample was originally completely uranium… 1 half life later… 8 8 4 8 2 8 1 …of the sample was uranium Now only 4/8 of the uranium remains – the other 4/8 is lead Now only 2/8 of uranium remains – the other 6/8 is lead Now only 1/8 of uranium remains – the other 7/8 is lead 8 So it must have taken 3 half lives for the sample to decay until only 1/8 remained (which means that there is 7 times as much lead). Each half life is 4, 000 years so the sample is 12, 000 years old.

Potassium decays into argon. The half life of potassium is 1. 3 billion years. A sample of rock from Mars is found to contain three argon atoms for every atom of potassium. How old is the rock? The rock must be 2 half lives old – 2. 6 billion years

Radioactive substances emit radiation from the nuclei of their atoms all the time. The half-life of a radioactive isotope is Either the time it takes for the number of nuclei of the isotope in a sample to halve or the time it takes for the count rate from a sample containing the isotope to fall to half its initial level.

Types of Radiation

What is radioactive decay?

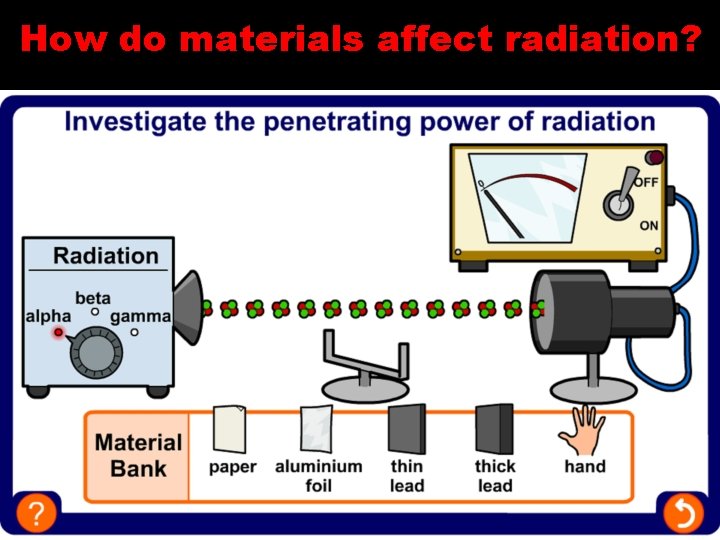
How do materials affect radiation?

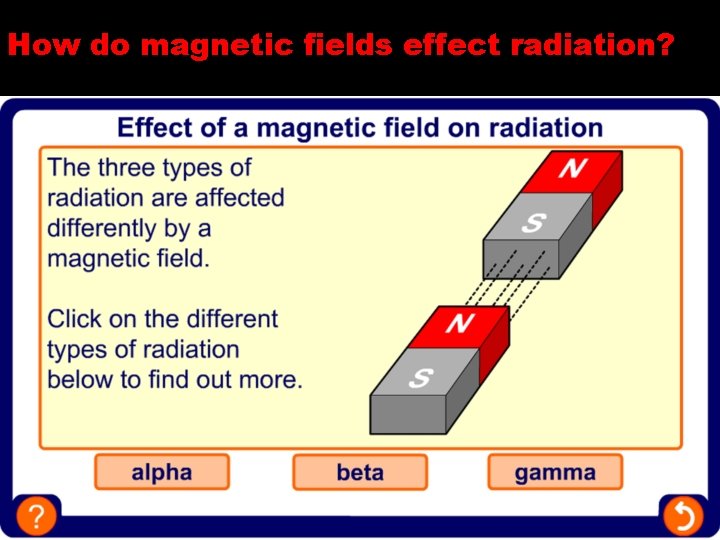
How do magnetic fields effect radiation?

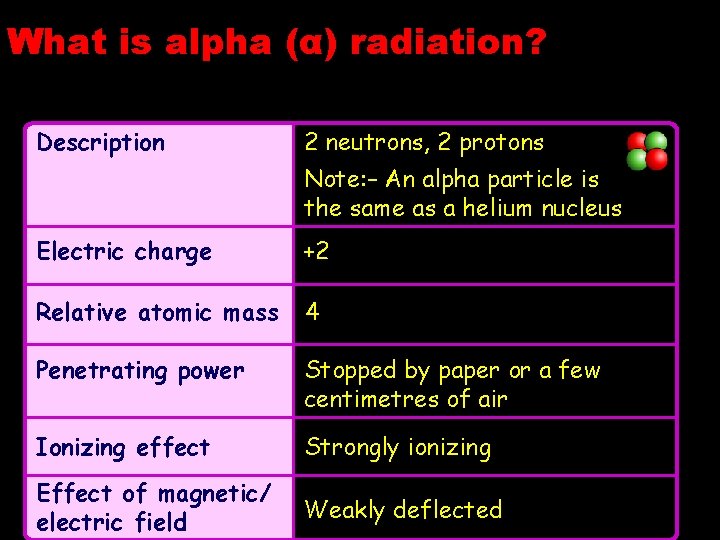
What is alpha (α) radiation? Description 2 neutrons, 2 protons Note: – An alpha particle is the same as a helium nucleus Electric charge +2 Relative atomic mass 4 Penetrating power Stopped by paper or a few centimetres of air Ionizing effect Strongly ionizing Effect of magnetic/ electric field Weakly deflected

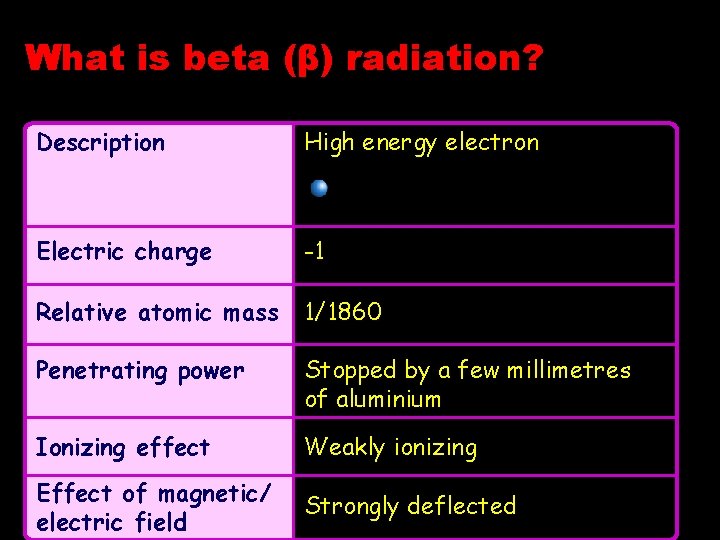
What is beta (β) radiation? Description High energy electron Electric charge -1 Relative atomic mass 1/1860 Penetrating power Stopped by a few millimetres of aluminium Ionizing effect Weakly ionizing Effect of magnetic/ electric field Strongly deflected

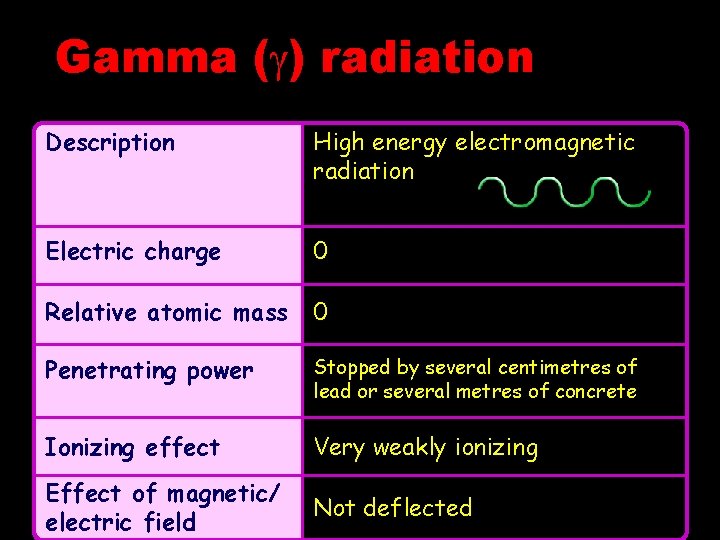
Gamma ( ) radiation Description High energy electromagnetic radiation Electric charge 0 Relative atomic mass 0 Penetrating power Stopped by several centimetres of lead or several metres of concrete Ionizing effect Very weakly ionizing Effect of magnetic/ electric field Not deflected

Types of radiation and penetrating power

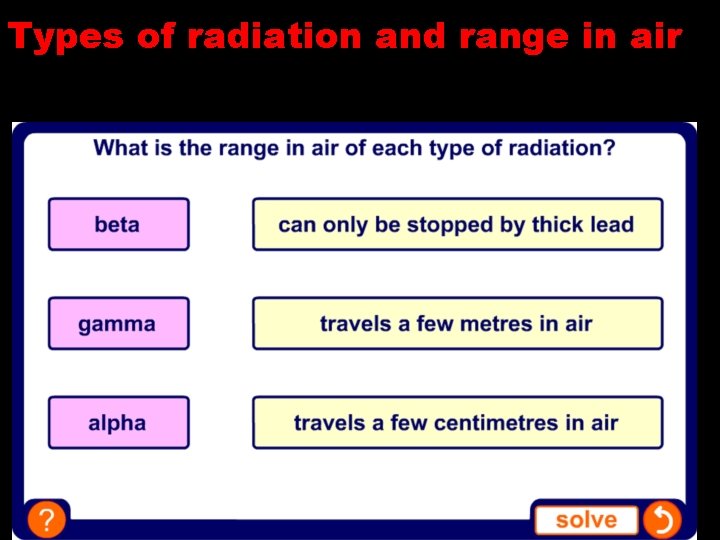
Types of radiation and range in air

Uses of Radiation

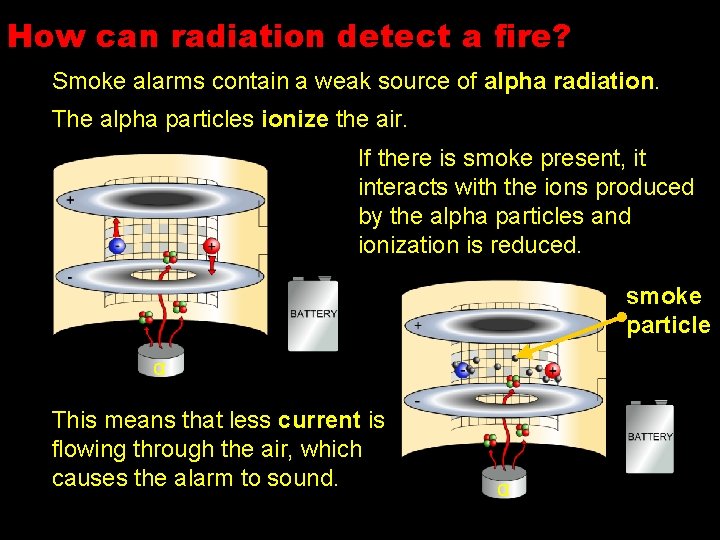
How can radiation detect a fire? Smoke alarms contain a weak source of alpha radiation. The alpha particles ionize the air. If there is smoke present, it interacts with the ions produced by the alpha particles and ionization is reduced. smoke particle α This means that less current is flowing through the air, which causes the alarm to sound. α

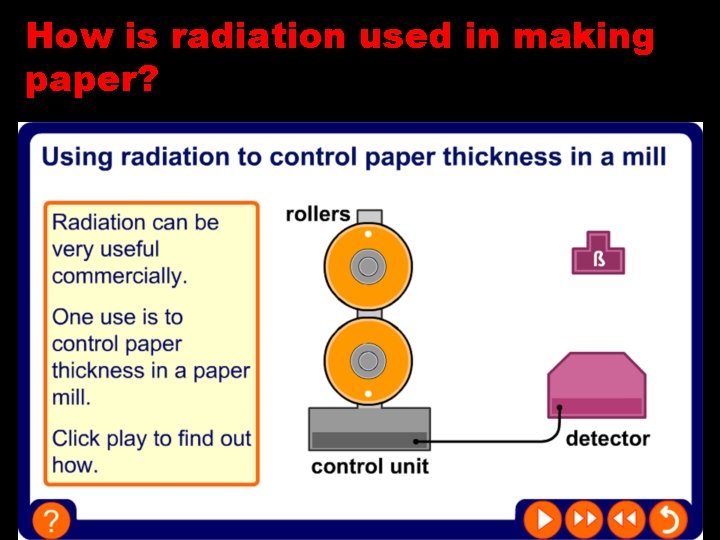
How is radiation used in making paper?

How can radiation find leaks in pipes?

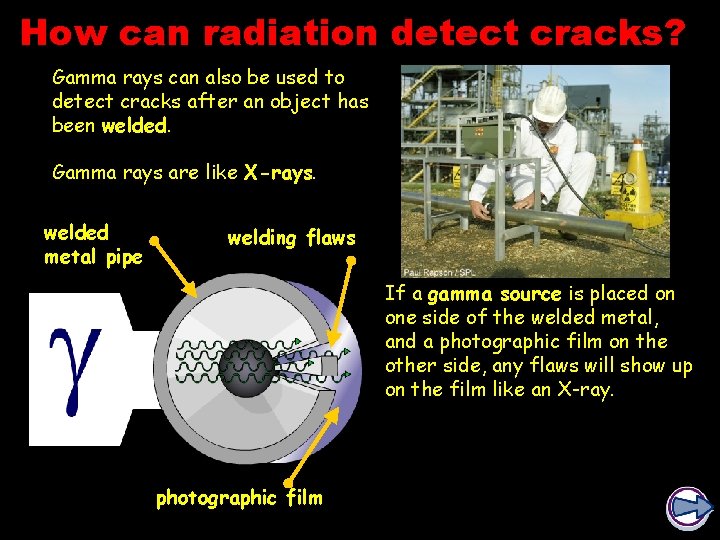
How can radiation detect cracks? Gamma rays can also be used to detect cracks after an object has been welded. Gamma rays are like X-rays. welded metal pipe welding flaws If a gamma source is placed on one side of the welded metal, and a photographic film on the other side, any flaws will show up on the film like an X-ray. photographic film

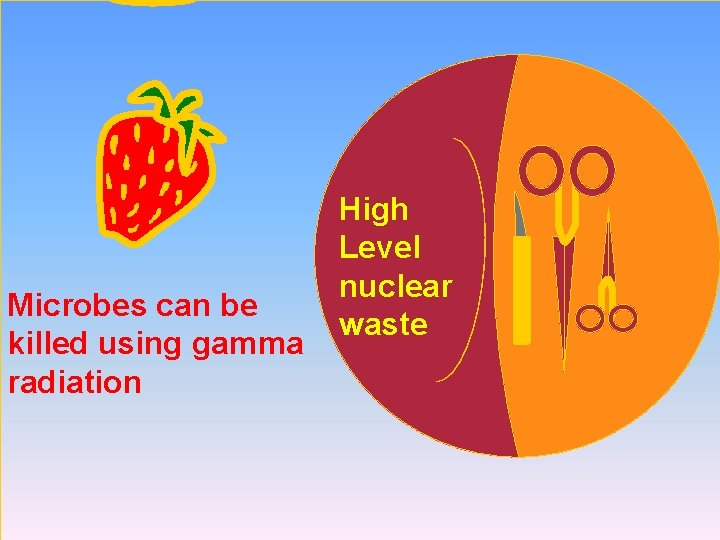
Microbes can be killed using gamma radiation High Level nuclear waste

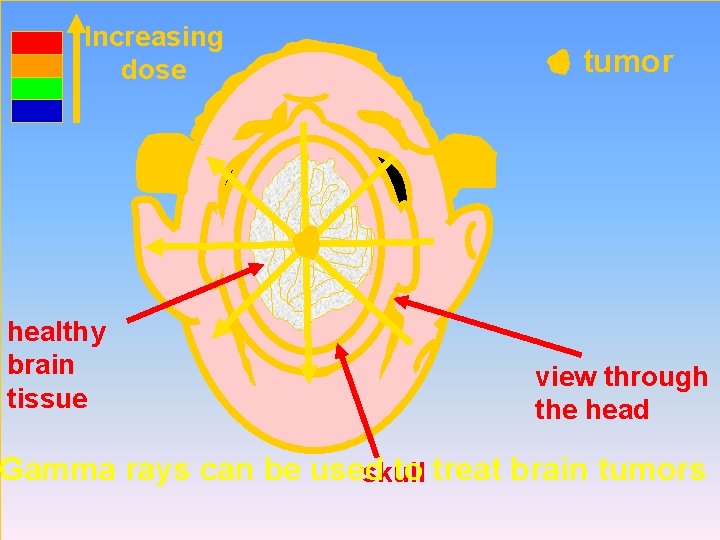
Increasing dose healthy brain tissue tumor view through the head Gamma rays can be used to treat brain tumors skull

Uses of radiation

Dangers of ionizing radiations

Radiation safety The three types of radiation differ in their effects and physical nature. All radioactive sources must be handled safely. The hazard symbol for radiation is shown below: As well as the normal laboratory safety rules you follow, are there any extra rules concerning radioactivity?

Radiation safety measures Radioactive materials could be very dangerous to handle if no safety precautions were taken. This is because people and their clothing could become contaminated. The safety precautions are: Write down on first side of the paper l keep exposure times as short as possible l monitor exposure with a film dose badge l label radioactive sources clearly l store radioactive sources in shielded containers l wear protective clothing l use tongs or a robotic arm to handle radioactive materials.

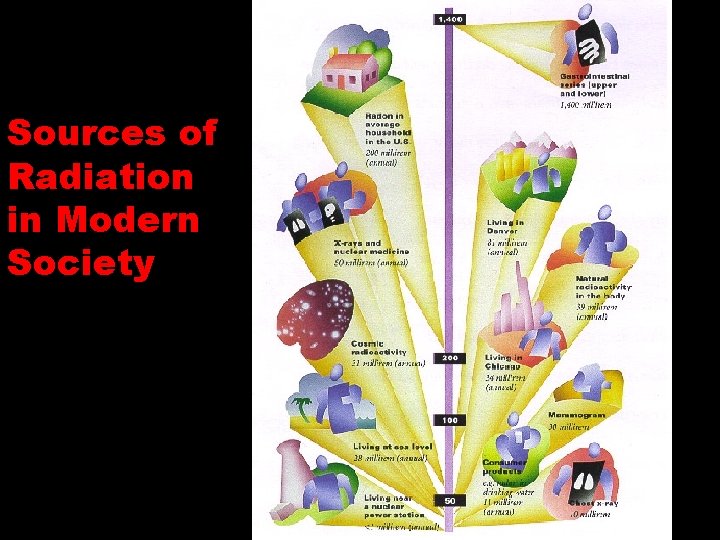
Background radiation is the radiation all around us. Most of the radioactivity you are exposed to is from natural sources. How many different sources of background radiation can you think of?

Sources of Radiation in Modern Society

Calculating background radiation