Haemophilus Haemophilus Gramnegative bacilli liking blood as per

Haemophilus
Haemophilus • Gram-negative bacilli liking blood (as per genus name) • Obligate Parasites of Man and Animals • Major pathogens for which humans are natural hosts v. Haemophilus influenzae üAcute pyogenic, normally invasive infections v. Haemophilus ducreyi üTrue pathogen (i. e. , not found in healthy individuals) üSTD; Soft chancre (chancroid)
Haemophilus species of clinical importance 1. H. influenzae -type b is an important human pathogen 2. H. ducreyi -sexually transmitted pathogen (chancroid) 3. Other Haemophilus are normal flora - H. parainfluenzae – pneumonia & endocarditis - H. aphrophilus – pneumonia & endocarditis - H. aegyptius – pink eye (purulent conjunctivitis)
Haemophilus Influenzae Aerobic gram-negative bacteria l Polysaccharide capsule l Six different serotypes (a-f) of polysaccharide capsule l 95% of invasive disease caused by type b (Hib) l
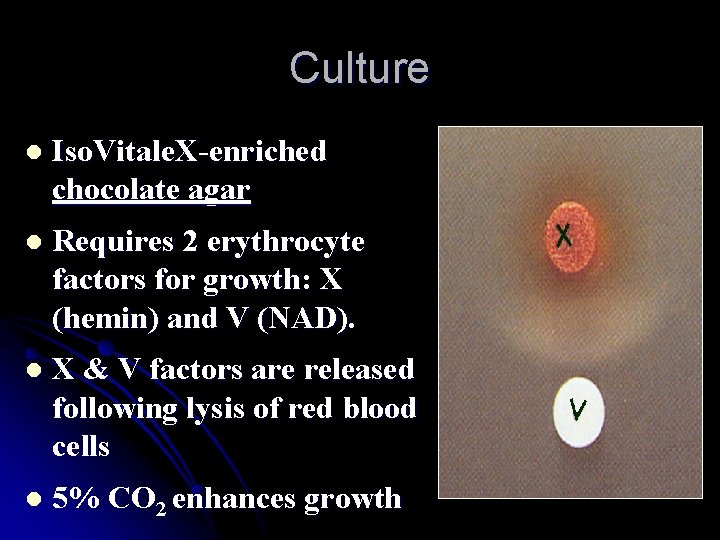
Culture l Iso. Vitale. X-enriched chocolate agar l Requires 2 erythrocyte factors for growth: X (hemin) and V (NAD). l X & V factors are released following lysis of red blood cells l 5% CO 2 enhances growth
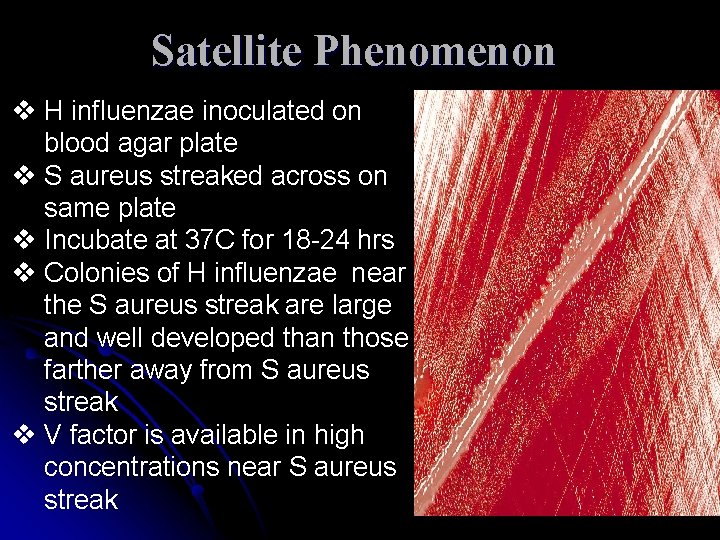
Satellite Phenomenon v H influenzae inoculated on blood agar plate v S aureus streaked across on same plate v Incubate at 37 C for 18 -24 hrs v Colonies of H influenzae near the S aureus streak are large and well developed than those farther away from S aureus streak v V factor is available in high concentrations near S aureus streak

Virulence factors l Antiphagocytic polysaccharide capsule is the major pathogenesis factor l Lipopolysaccharide lipid A component from the cell wall (major role in non capsule strains) l All virulent strains produce neuraminidase and an Ig. A protease

Clinical Presentation Meningitis: Fever, headache, nausea, vomiting, stiff neck, photophobia, seizures, coma; and in infants, poor feeding and a bulging fontanelle. Epiglottis: Sudden onset of sore throat, fever, and shortness of breath, progressing rapidly to difficulty swallowing and pooling and drooling of saliva due to the obstructed airway.

Clinical Presentation l Pneumonia: Severe shortness of breath, rapid heart rate, fever, cough and evidence of pneumonia by chest radiograph. l Septic Arthritis: Swelling, warmth, pain with movement and decreased mobility of a single large weight-bearing joint.

Laboratory diagnosis Specimens: CSF, Blood, Throat swab, Swab, Pus l H influenzae is sensitive to low temperatures, hence specimens should never be refrigerated. l Microscopy: Grams stain, Immunofluorescence, Quellungs reaction for capsule demonstration l Antigen detection: Latex agglutination, Coagglutination, CIE l Culture: l
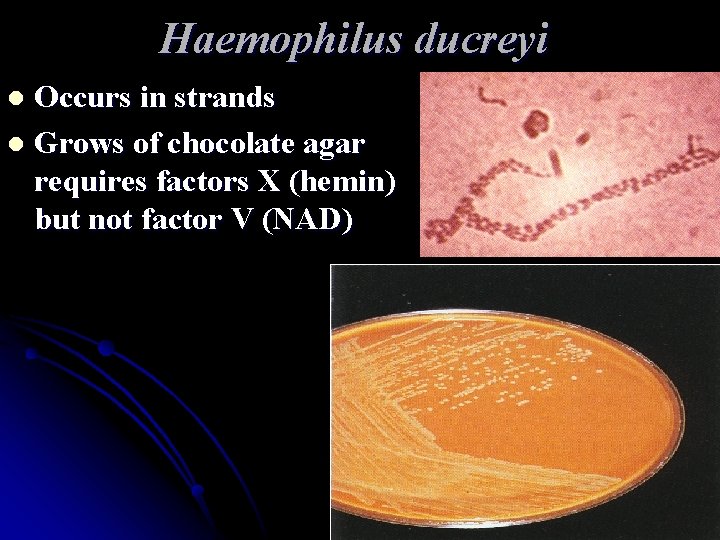
Haemophilus ducreyi Occurs in strands l Grows of chocolate agar requires factors X (hemin) but not factor V (NAD) l
Chancroid STD transmission l papule, non indurated painful ulcer, enlarged lymph nodes (bubos) l Lab diagnosis l Smear: Gramnegative bacilli (school of fish) bipolar staining. l Culture: chocolate agar with 1% Isovitalex, 33 C°, 7 days, only X factor l
Bordetella
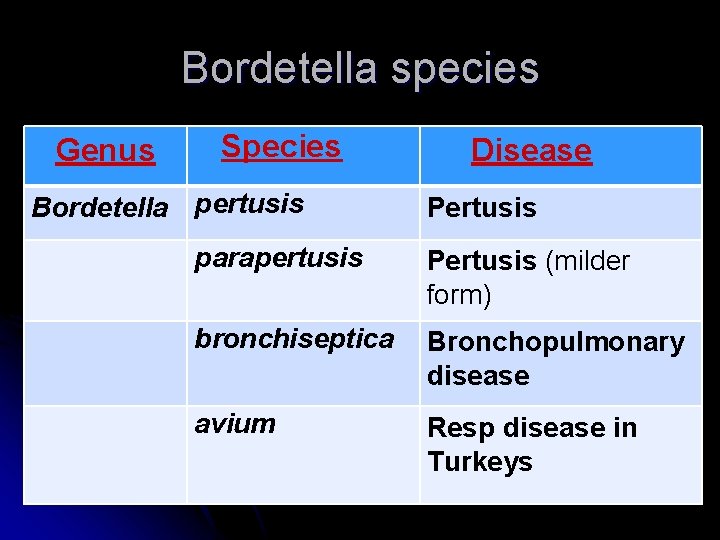
Bordetella species Genus Species Bordetella pertusis Disease Pertusis parapertusis Pertusis (milder form) bronchiseptica Bronchopulmonary disease avium Resp disease in Turkeys
Bordetella pertusis Aerobic, Gram negative coccobacillus l Specific to Humans l Colonizes the respiratory tract l l Whooping Cough (Pertussis)

Virulence Factors Adhesions l Toxins l
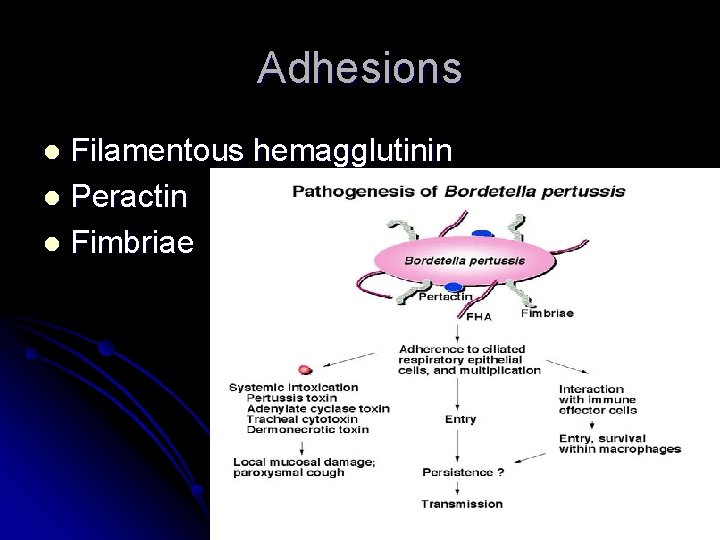
Adhesions Filamentous hemagglutinin l Peractin l Fimbriae l

Toxins Pertusis Toxin l Adenylate Cyclase Toxin l Tracheal cytotoxin l Heat-labile toxin l
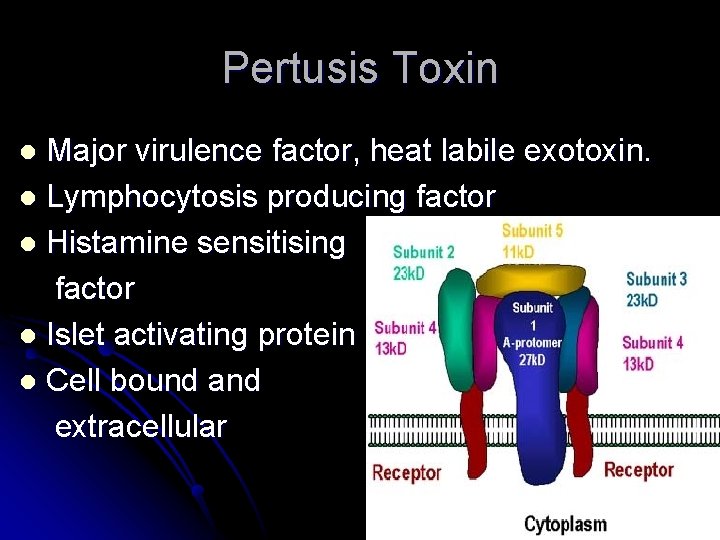
Pertusis Toxin Major virulence factor, heat labile exotoxin. l Lymphocytosis producing factor l Histamine sensitising factor l Islet activating protein l Cell bound and extracellular l

Adenylate Cyclase Toxin Invasive toxin l Activated by host cell calmodulin l Impairment of immune effector cells l

Whooping Cough Also known as Pertusis l Mainly transmitted by droplets. l Major cause of childhood fatality prior to vaccination l
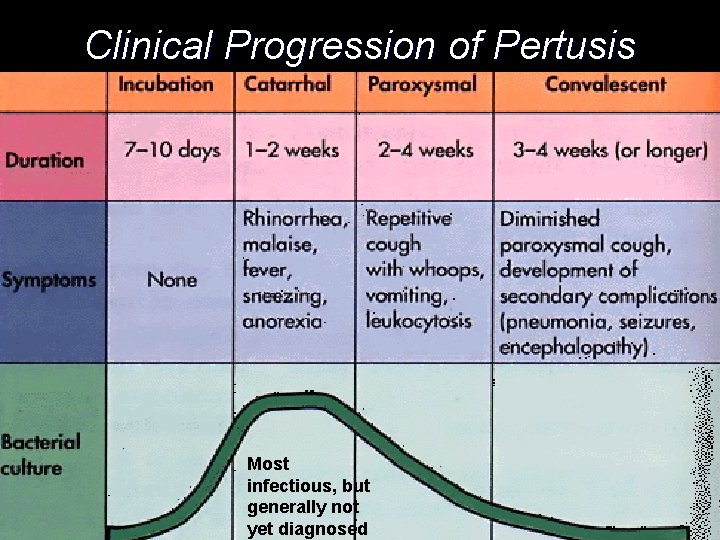
Clinical Progression of Pertusis Most infectious, but generally not yet diagnosed

Pertusis complications Subconjuntival hemorrhages due to violent bouts of cough l Bronchopneumonia and lung collapse l Convulsions and coma l

Diagnosis Isolation by culture l Serological testing l

Culture The pernasal swab l The cough plate method l The postnasal (Peroral) swab l Media: Bordet- Gengou (glycerol-potatoblood agar) medium. v Small, smooth, opaque, greyish white, resembling “bisected pearls or mercury drops”. l

Serology Rising titre of antibodies l 3 rd week of illness l Specific Ig. A in nasopharyngeal secretions by ELISA l
Pertusis Vaccine 1 st Pertusis vaccine- whole cell l Acellular vaccine now used l Combination vaccines l Triple vaccine: DTP (Diphtheria, Pertusis, Tetanus) at 6, 10 and 14 weeks of age. Booster doses at 2 ½ years. l

Brucella

Brucella • Brucella is named after Sir David Bruce, who in 1886 isolated the causative agent from a soldier in Malta. • Brucella species are recognized based on the natural animal host to the following species
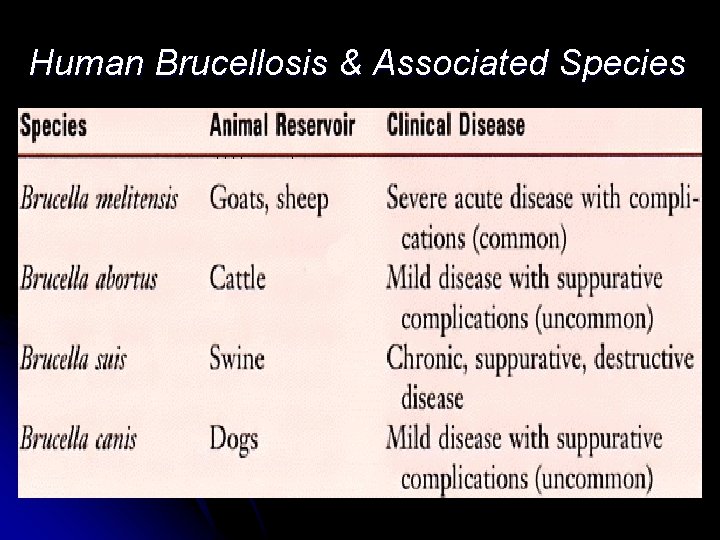
Human Brucellosis & Associated Species
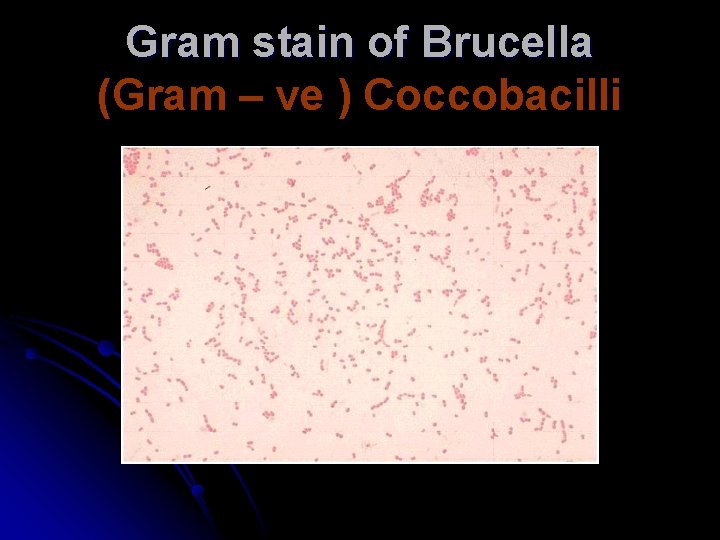
Gram stain of Brucella (Gram – ve ) Coccobacilli

Culture Strict aerobes, B abortus is capnophilic l Can grow on ordinary media. l Media employed: Blood agar, serum. Dextrose agar, Trypticase soy agar, serum -potato-infusion agar l
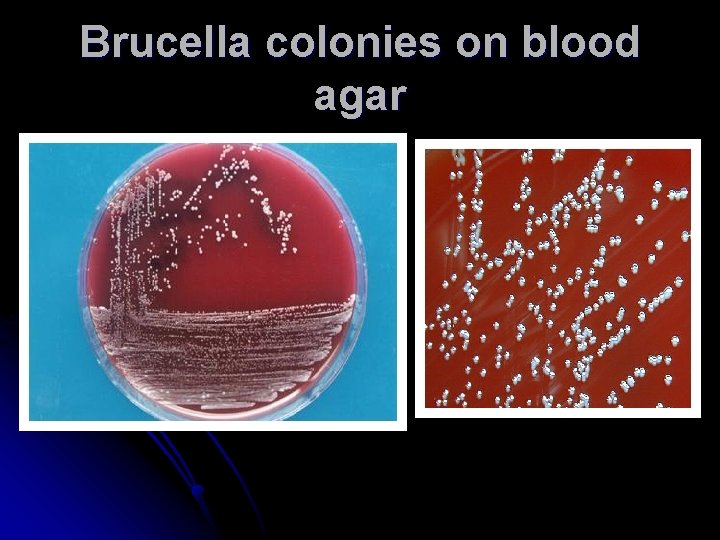
Brucella colonies on blood agar

Growth in presence of aniline dyes Basic fuschin, Thionin l Br. Melitensis: not inhibited by both dyes l Br abortus: thionin(-), basic fuschin (+) l Br suis: thionin(+), basic fuschin (-) l

Mode of infection Drinking unpasteurized milk l Direct contact with infected animal tissues, dairy farmers, veterinarians l Laboratory personnel exposed to cultures l

Types of infection Subclinical or latent infection l Acute brucellosis l Chronic brucellosis l

Clinical Presentation of Human Brucellosis • Acute disease often develops with initial • • nonspecific symptoms of malaise, chills, fatigue, weakness, myalgias (muscles), weight loss, arthralgias (joint), hepatosplenomegaly. Undulant fever: periodic nocturnal fever, occurring over weeks, months or years Chronic disease (> 6 months): Ability to survive in phagocytic cells and multiply to high concentrations
Laboratory diagnosis Blood culture l Isolation from liver, lymphnode, bone marrow etc l

Blood culture Gold standard l Castaneda’s method of blood culture used to avoid contamination during subcultures l Trypticase soy broth used l Blood culture should be incubated for atleast 8 weeks l

Serology Antibodies appear in 7 -10 days 1. Standard tube agglutination test: Ig. M 2. Complement fixation test: Ig. M and Ig. G 3. ELISA & RIA 4. Indirect immunofluorescence test 5. Castaneda’s strip test l
Standard tube agglutination test Detects Ig. M l Serial serum dilutions 1: 20 to 1: 640 l Standardised heat killed brucella antigen is added l Single titre of > 1: 160 is significant l Prozone phenomenon to be avoided l

Other tests Brucellin test: Delayed hypersensitivity test to brucella antigen. Induration of >6 mm within 24 hrs is positive l Detection of animal infection: v Milk ring test: sample of whole milk+ stained (Hematoxylin) brucella antigen. Incubated at 70 c for 40 -50 min ü Coloured ring at top: positive ü Uniformly stained milk: negative l
- Slides: 43