Haematopoiesis formation of blood cells 1 Haematopoiesis aka

Haematopoiesis: formation of blood cells 1

Haematopoiesis • aka haemopoiesis • and nb if US spelling, hemo- 2
Haematopoiesis • This is the process by which ALL blood cells (= haematological system) are produced – platelets, red blood cells, leukocytes of all sorts – (AND probably also endothelium). 3
Haematopoiesis • Haematopoiesis is a highly organised differentiation process involving the ordered expression of different sets of genes • It is controlled by factors in the environment of the developing blood cell – the bone marrow in adults • just like any other developmental programme. 4

The haematopoietic stem cell • Mature blood cells in healthy individuals mostly have short lifetimes (exception: lymphocytes) and are constantly regenerated in the bone marrow. – we make 5 x 1011 blood cells daily • This is accelerated when there is haematological stress – e. g. infection, need more leukocytes – e. g. high altitude, need more red cells. 5
Haematopoiesis • Haematopoiesis begins at a very early stage in embryonic development, at about 3 weeks in the human. • At that time the cells in the embryo separate into 2 sets, one generating the embryo proper & all the tissues of the adult, the other forming the YOLK SAC (aka vitelline sac) which is the site where blood cells and blood vessels are first 6 formed.
Haematopoiesis • The yolk sac is an ovoid structure joined to the embryo by a stalk. • It contains mesoderm derived cells – “haemangioblasts” • which differentiate to form (nucleated) red blood cells and endothelial cells which generate a capillary system (“plexus”) within the yolk sac. 7

Haematopoiesis • At the same time the heart and aorta start to form: these join up with the capillary plexus and the erythrocytes start to circulate. 8
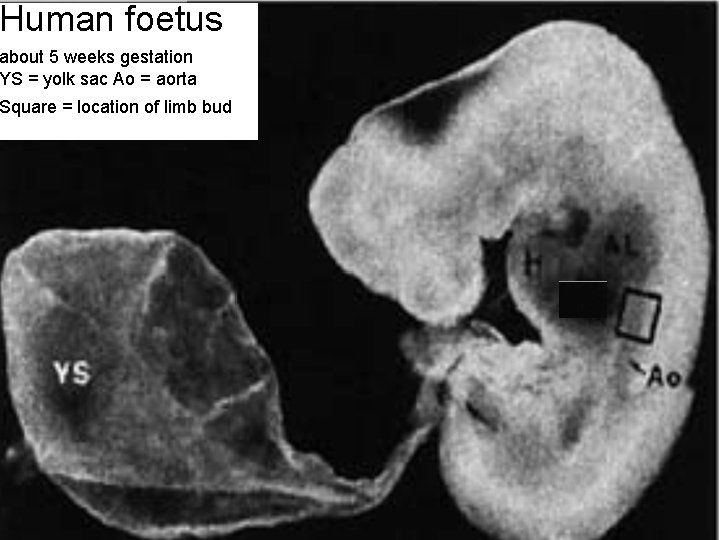
Human foetus about 5 weeks gestation YS = yolk sac Ao = aorta Square = location of limb bud 9

Definitive haematopoiesis 10
Haematopoiesis • At a later stage of embryogenesis (after about week 6 in humans) haematopoiesis occurs mainly in the liver and at birth shifts to the bone marrow (BM). • This haematopoiesis is described as definitive unlike the early primitive haematopoiesis occurring in the yolk sac. • Now, the entire range of blood cells found in the adult are produced. 11
Haematopoiesis • The BM is in effect a highly specialised tissue comprising a range of cells – some of which (haematopoietic cells) form blood cells – others (stromal cells) provide a support functions for the haematopoietic cells, providing the specialised environment needed for haematopoiesis to occur. – Yet other cells (osteoblasts and osteoclasts) are concerned with producing the bone itself. 12
Haematopoiesis (Bear in mind that cells of the immune system undergo further proliferation and differentiation in the periphery – especially in secondary lymphoid tissue – during immune responses: the purpose of haematopoiesis is to generate cells which are capable of responding to pathogens. ) 13

BONES: where haematopoiesis occurs 14

dem bones – more than a framework to hang muscles from 15
bones • Source of skeletal rigidity • reservoir of Ca 2+ and PO 43 • haematopoietic organs. 16
bones • Bone is a specialised form of connective tissue (mesodermal/mesenchymal origin) with an extracellular matrix (ECM) which is rigid http: //www. emedicine. com/orthoped/TOPIC 403. HTM • Rigid outer layer of dense compact (aka cortical) bone, 70% hydroxyapatite (hydrated calcium phosphate) • Inner core of much less dense spongy bone (aka cancellous, trabecular). 17
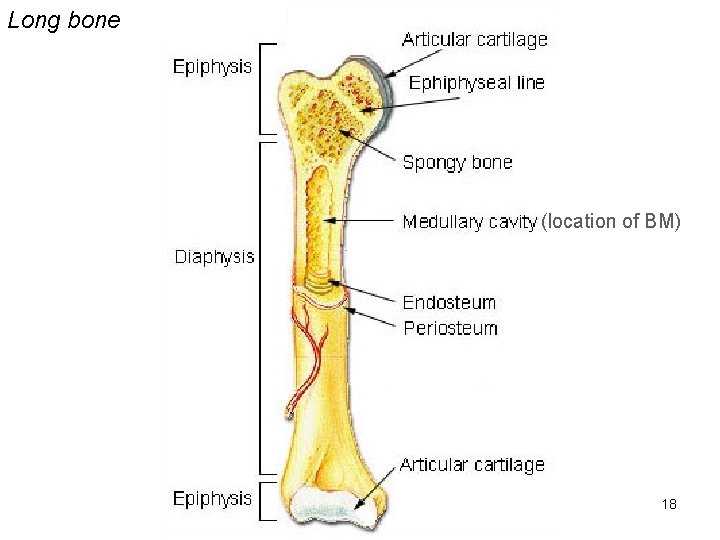
Long bone (location of BM) 18

Schematic of cross section of long bone contains blood vessels nerves and lymphatics http: //en. wikipedia. org/wiki/Bone joins together Haversian canals The osteon is the unit structure of compact bone made up of concentric mineralised lamellae around a central (Haversian) canal. 19
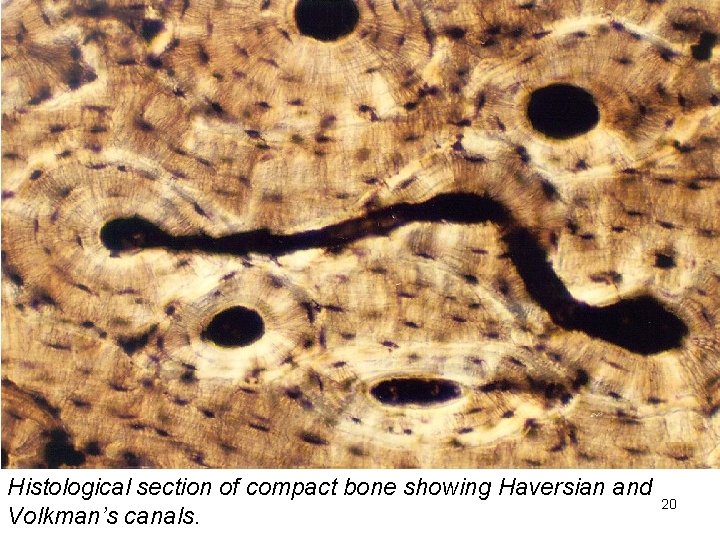
Histological section of compact bone showing Haversian and Volkman’s canals. 20

bones • The bone marrow (BM) is within the medullary cavity especially of long bones. • As mentioned it is intensely cellular because that is where blood cells are produced. 21
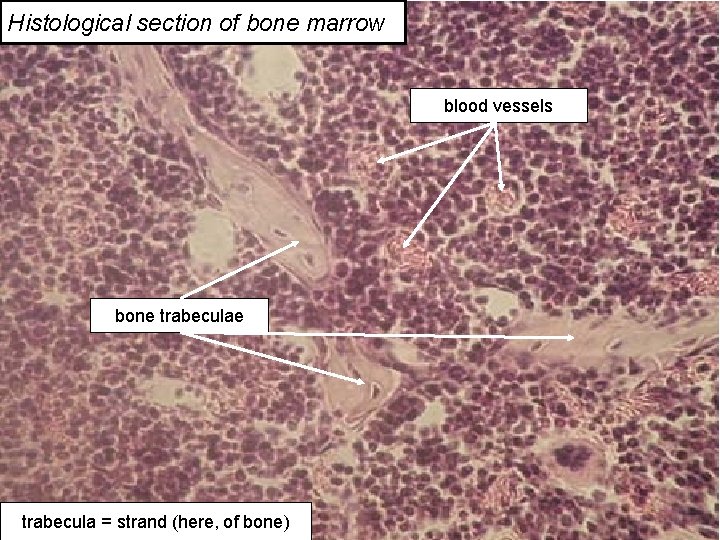
Histological section of bone marrow blood vessels bone trabecula = strand (here, of bone) 22

bones • Bones always start (in development) from cartilage: this is converted to bone by deposition of hydroxyapatite (a form of calcium phosphate) by osteoblasts. • But throughout life, bone tissue is renewed – this is due to a balance between degradation due to osteoclasts and production due to osteoblasts. 23
bones • Osteoblasts (a type of BM stromal cell) are responsible for laying down a collagen matrix (osteoid) & they subsequently mineralise this with hydroxyapatite. • They are activated by growth factors e. g. bone morphogenic protein (BMP) & certain steroid hormones e. g. oestrogens. 24

bones • Osteoclasts (derived from haematopoietic cells) resorb bone. • They are activated by certain cytokines e. g. IL-6. • They secrete acids which dissolve the hydroxyapatite and proteases which degrade the collagen. 25

bones • This balance between production and degradation can be disturbed in some diseases where the osteoclasts get the upper hand – e. g. osteoporosis due to low levels of oestrogen, myeloma due to high levels of IL-6. 26
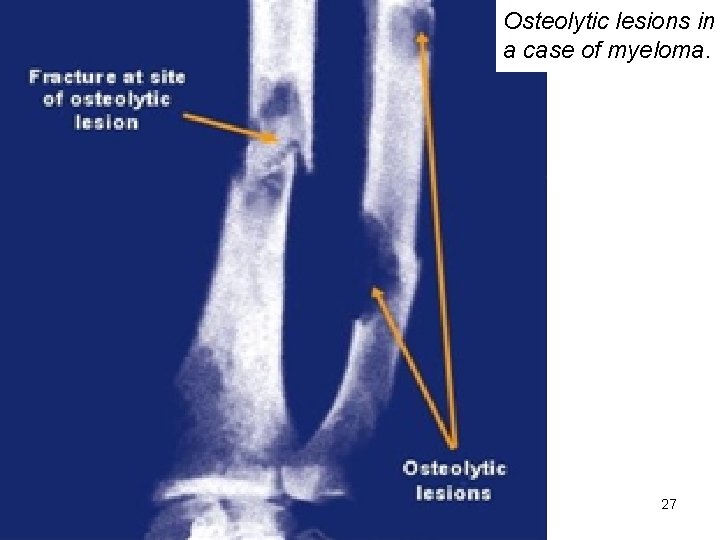
Osteolytic lesions in a case of myeloma. 27

The haematopoietic stem cell: source of all blood cells 28
The haematopoietic stem cell • Haematopoietic stem cells (HSCs) are the parental cell type for ALL blood cells – and probably also endothelium. • HSCs are (usually) present only in the bone marrow – also present in cord blood and in (adult) peripheral blood after treatment with “mobilising” cytokines. 29
The haematopoietic stem cell • The HSC is (probably) able to divide indefinitely, or at least a very large number of times – experiments in 1960 s in which mice were irradiated or treated with drugs to destroy their bone marrow showed that transplantation of very small numbers of bone marrow (BM) cells could “reconstitute” the mouse – serial transplant could be done. 30

The haematopoietic stem cell • Experiments in the 1970 s showed that all blood cell types could be derived from single BM cells. 31
The haematopoietic stem cell • These depended on irradiating the BM cells used to reconstitute the mice with SMALL doses of radiation, not enough to kill the cells but enough to cause minor chromosome alterations (“chromosomal markers”) in rare cells – each marker unique; – all descendents of a marked cell (a CLONE) have the same maker. 32
The haematopoietic stem cell • In some individual transplanted mice there could be found cells of all leukocyte types bearing the same marker • hence a single pluripotent stem cell present in the transplant was capable of giving rise to all lineages. – (Probably not totipotent i. e. cannot make all body cells. ) 33

The haematopoietic stem cell • In other mice, ALL myeloid but NOT lymphoid cells had the same marker • And in yet others ALL lymphoid but NOT myeloid cells had the same marker. 34
The haematopoietic stem cell • This implied that there were also stem cells of more restricted potency – capable of producing EITHER myeloid OR lymphoid cells – these are now known as common lymphoid or myeloid progenitor cells – CLP & CMP. 35
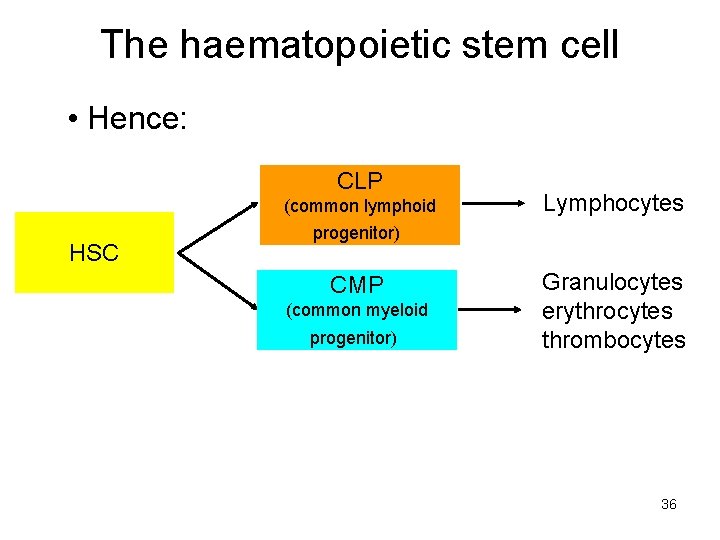
The haematopoietic stem cell • Hence: CLP (common lymphoid HSC Lymphocytes progenitor) CMP (common myeloid progenitor) Granulocytes erythrocytes thrombocytes 36
The haematopoietic stem cell • Stem cell self renewal: – evidently since mature leukocytes die the HSC must be able to proliferate at least over the lifetime of the individual. – This proliferation must be assymetric to generate a progenitor cell (CMP or CLP) which has more limited differentiation capacity AND replace the stem cell. 37
The haematopoietic stem cell • CMPs and CLPs are transient amplifying (TA) cells & proliferate PRIOR TO further differentiation (i. e. before they become granulocytes or whatever) – this is a limited proliferation, increasing cell numbers. 38

The haematopoietic stem cell CMP proliferation of progenitors to amplify numbers (or CLP) CMP CMP CMP HSC CMP asymmetric division to generate progenitor cell & replace HSC 39
The haematopoietic stem cell • What is the HSC? – A rare (<0. 1%) cell present in bone marrow expressing the antigen CD 34. – We now know that CD 34+ cells are the essential cell type for BONE MARROW TRANSPLANTATION. – Other cells express CD 34 (e. g. endothelial cells) so it us not a unique marker for HSCs. 40
The haematopoietic stem cell • It is argued that stem cells require a specialised environment to maintain the “stem-ness” of the stem cell: – the stem cell niche. • HSCs are found in two locations in BM: – the endosteum (boundary between solid bone & marrow) associated with osteoblasts; – the perivascular region around vascular sinusoids (large blood vessels with thin-walled comprised of fenestrated endothelium). 41
The haematopoietic stem cell • Hence “endosteal” and “vascular” niches. • Do they have different roles? – It has been suggested that the endosteal niche contains long-term, slowly dividing HSCs which maintain the vascular niche HSCs. – In the vascular niche the HSCs are more actively dividing and progenitor cells are produced. 42
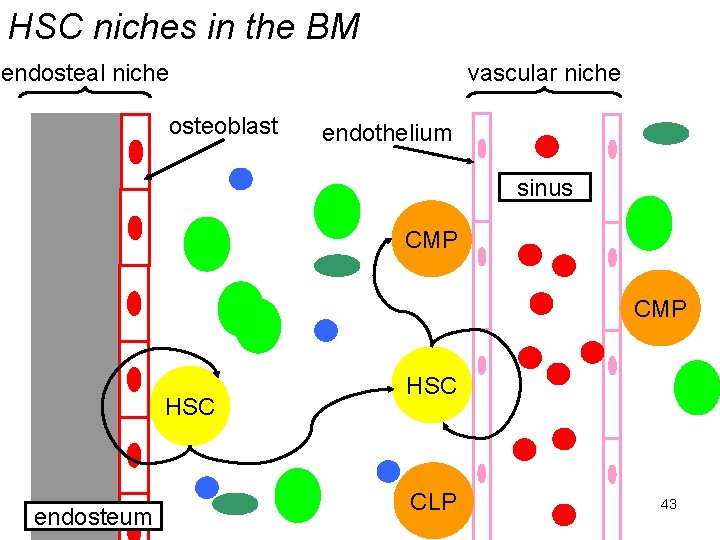
HSC niches in the BM endosteal niche osteoblast vascular niche endothelium sinus CMP HSC endosteum HSC CLP 43
The haematopoietic stem cell • It is not clear what molecules are involved in defining the stem cell niches – presumably adhesion molecules & diffusible factors produced by the non-HSC (“stromal”) cells – osteoblasts, endothelial cells etc – within the niche. 44
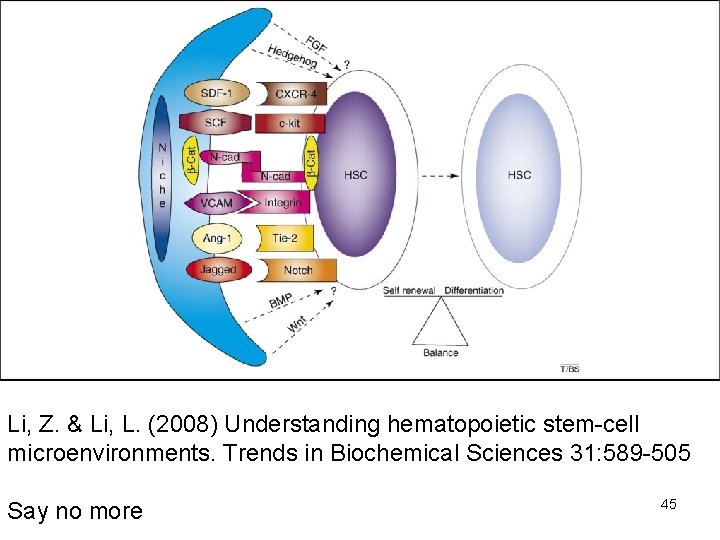
Li, Z. & Li, L. (2008) Understanding hematopoietic stem-cell microenvironments. Trends in Biochemical Sciences 31: 589 -505 Say no more 45
Progenitor cell growth and differentiation 46
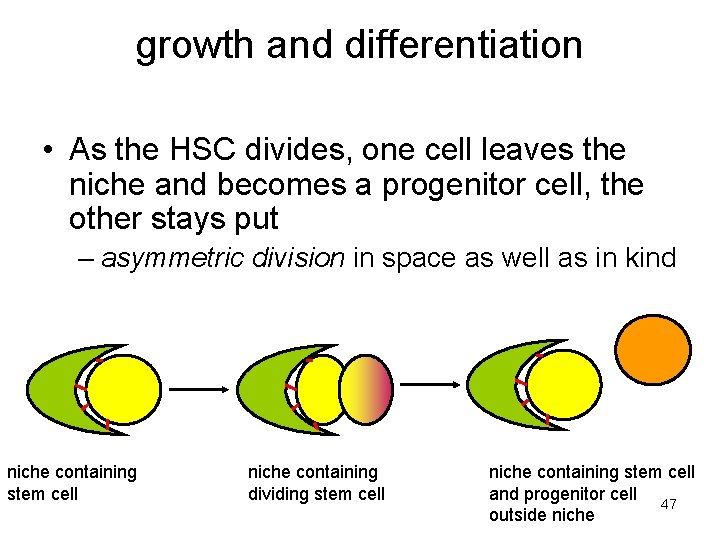
growth and differentiation • As the HSC divides, one cell leaves the niche and becomes a progenitor cell, the other stays put – asymmetric division in space as well as in kind niche containing stem cell niche containing dividing stem cell niche containing stem cell and progenitor cell 47 outside niche
growth and differentiation • Evidently the progenitor cell is now in a different environment. – Growth factors drive its growth and then differentiation. – (Perhaps the job of the stem cell niche is to slow cell division and block differentiation. ) – The different phases of differentiation and the growth factors required have been largely worked out by cell culture experiments. 48
growth and differentiation • In vitro culture of bone marrow cells – culture of BM cells is often done suspended in semi-solid media so that the progeny of a single cell can be seen as a colony. 49
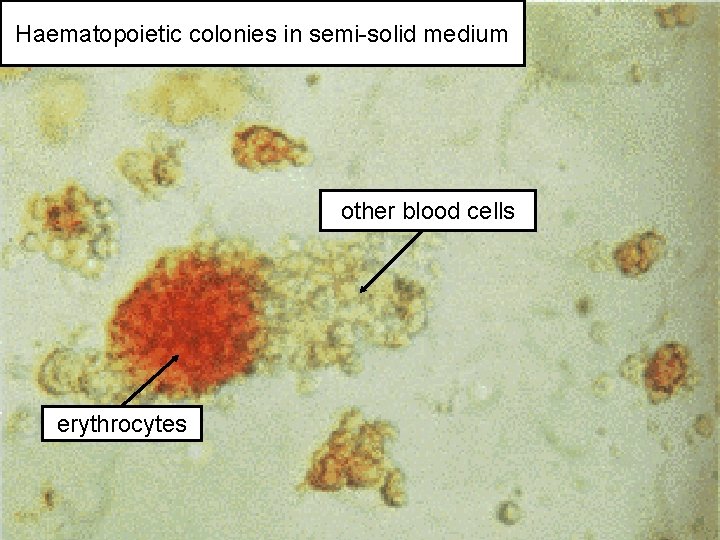
Haematopoietic colonies in semi-solid medium other blood cells erythrocytes 50
growth and differentiation • Alternatively, cells can be grown in liquid culture – this requires the presence both of haematopoietic cells and BM stromal cells to recreate something like the stem cell niche – even so cell growth is very inefficient. 51
growth and differentiation • In both cases added growth factors & cytokines are needed to drive both proliferation and differentiation of cells. 52
growth and differentiation • There are many of these, some more-orless specific for different cell types – e. g. Granulocyte/monocyte colony stimulating factor (GM-CSF), erythropoeitin (EPO) – NB the term CSF. • Others with much broader activities – e. g. IL-3 which will stimulate growth of most haematopoietic cell types. 53
growth and differentiation • Growth factors/cytokines act both in a paracrine fashion – they diffuse from the producer cell to the responder cell • and in a juxtacrine fashion – they remain on the cell surface of the producer cell and so the responder cell must be juxtaposed – touching. 54
growth and differentiation • And they are produced both within the BM and from sources outside the BM – e. g. GM-CSF from lymphocytes, EPO from kidney. • Infection can massively increase the amount of growth factors present because of activation of a range of leukocytes at the site of infection – hence increased haematopoiesis. 55

growth and differentiation • Adhesion interactions especially via integrins are also important in haematopoiesis • not just to drive growth & differentiation but also to prevent apoptosis. 56

erythrocytes & platelets 57
erythrocytes & platelets • The early stages for production of both these cells pass through a common pathway generating a “megakaryocyte/erthrocyte precursor” (MEP) from the CMP: they then split. 58
erythrocytes & platelets • erythropoietin (EPO: aka epoietin) produced by kidneys specifically stimulates erythropoiesis – EPO production stimulated by hypoxia – EPO binds to erythrocytes so haemorrhage or anaemia results in increased levels of EPO – these lead to stimulation of erythropoiesis. – (Other non-specific GFs/cytokines needed. ) 59
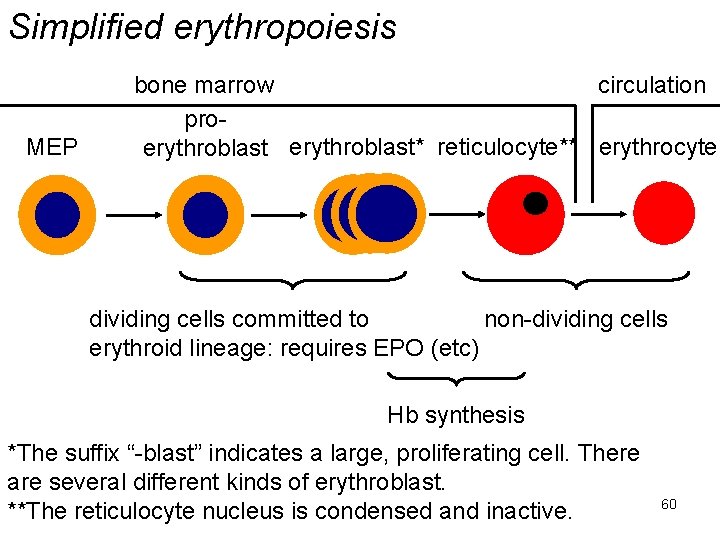
Simplified erythropoiesis MEP bone marrow circulation proerythroblast* reticulocyte** erythrocyte EP EP dividing cells committed to non-dividing cells erythroid lineage: requires EPO (etc) Hb synthesis *The suffix “-blast” indicates a large, proliferating cell. There are several different kinds of erythroblast. **The reticulocyte nucleus is condensed and inactive. 60

erythrocytes & platelets • Erythrocyte clearance: – removed by liver and spleen after 120 days – as the erythrocyte ages surface proteins particularly “band 3” are progressively oxidised – this provides a target for phagocytosis by macrophages lining liver & spleen sinuses. 61
erythrocytes & platelets • Generation of platelets (thrombopoiesis): – stimulated by thrombopoietin (TPO) & other non-specific growth factors. – TPO produced constitutively mostly by liver. – Inflammation can double production by liver via cytokine IL-6. – In thrombocytopenia (reduced platelets) BM stromal cells also produce TPO. – Platelets have TPO receptors and so remove TPO from circulation (-ve feedback). 62
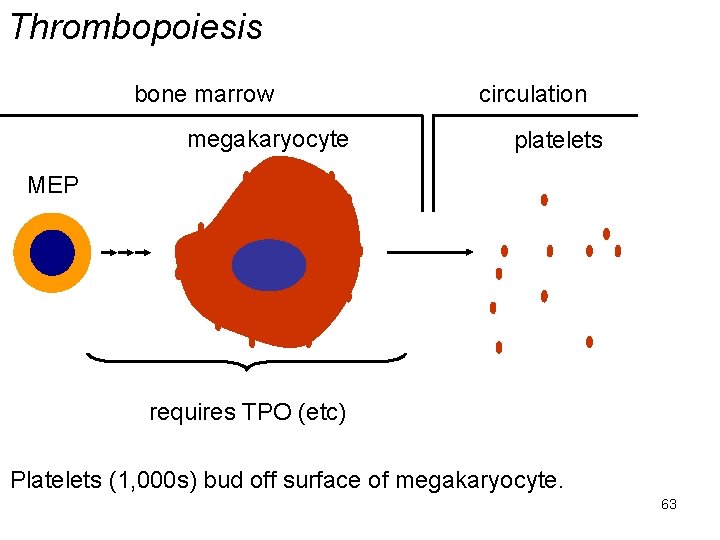
Thrombopoiesis bone marrow megakaryocyte circulation platelets MEP requires TPO (etc) Platelets (1, 000 s) bud off surface of megakaryocyte. 63

erythrocytes & platelets • Platelet clearance: – removed by liver and spleen after ~ 7 days. 64

Granulocytes and monocytes 65
granulocytes and monocytes • Granulocytes (basophils, mast cells, eosinophils & neutrophils) and monocytes share early steps in differentiation via “granulocyte/monocyte precursor” (GMP) derived from the CMP. • Formation of GMP from CMP is driven by granulocyte/monocyte colony stimulating factor (GM-CSF) (etc). 66
granulocytes and monocytes • Formation of specific cell types is driven by other growth factors, all of which are produced constitutively and are induced e. g. by inflammation. 67
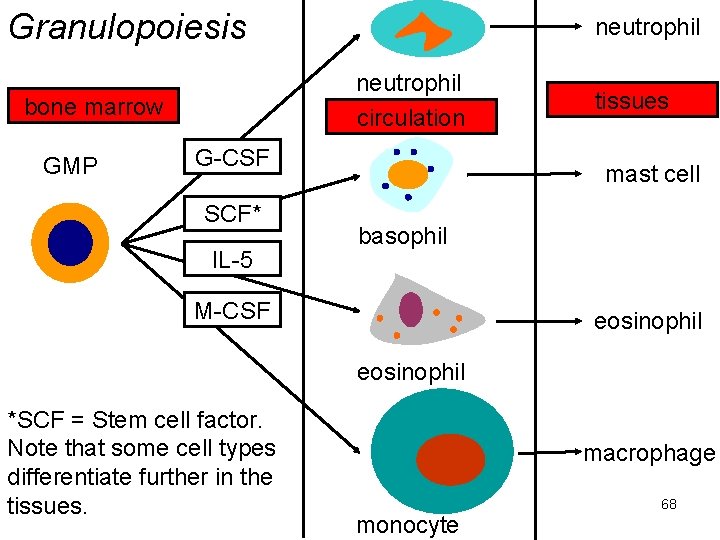
Granulopoiesis neutrophil circulation bone marrow GMP neutrophil G-CSF SCF* IL-5 tissues mast cell basophil M-CSF eosinophil *SCF = Stem cell factor. Note that some cell types differentiate further in the tissues. macrophage monocyte 68
granulocytes and monocytes • Most of these cells are relatively short lived and are lost by apoptosis – the fragments of the dead cells are phagocytosed by cells lining sinuses of spleen & BM – or, in the tissues, by epithelial cells. 69

lymphocytes 70
lymphocytes • Two pathways: – T cells – B cells and NK cells • Both from CLP • Initial stages in BM: B & NK cells complete maturation in BM, immature T cells migrtae to thymus and mature there. 71
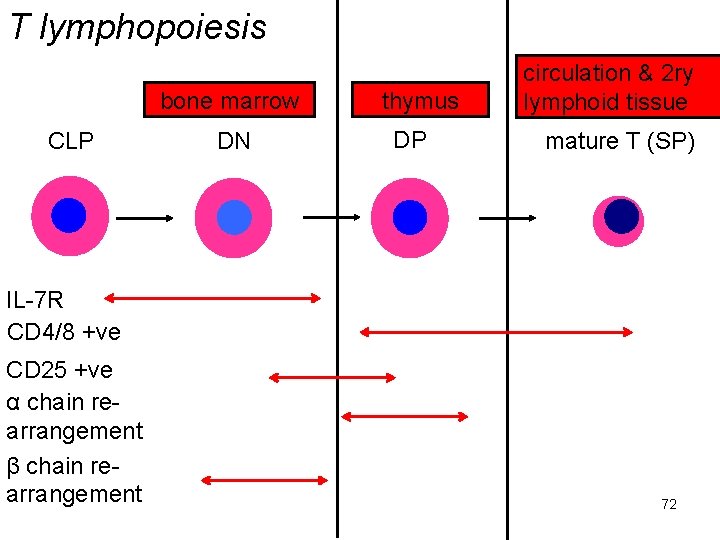
T lymphopoiesis bone marrow CLP DN thymus DP circulation & 2 ry lymphoid tissue mature T (SP) IL-7 R CD 4/8 +ve CD 25 +ve α chain rearrangement β chain rearrangement 72
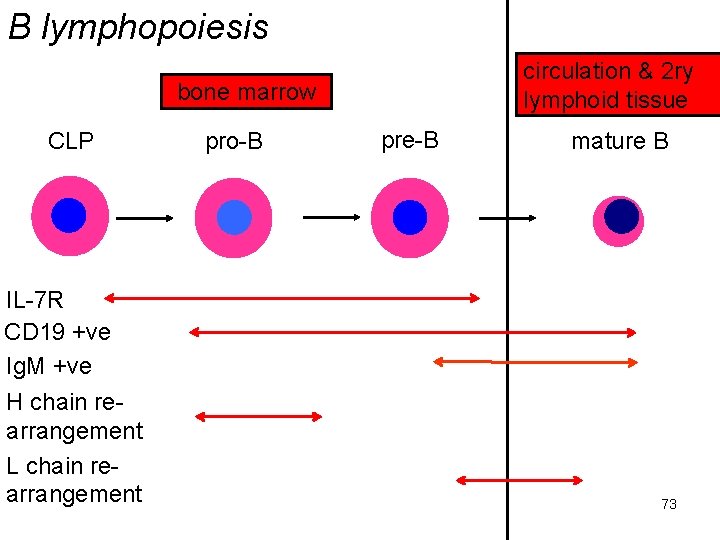
B lymphopoiesis circulation & 2 ry lymphoid tissue bone marrow CLP IL-7 R CD 19 +ve Ig. M +ve H chain rearrangement L chain rearrangement pro-B pre-B mature B 73
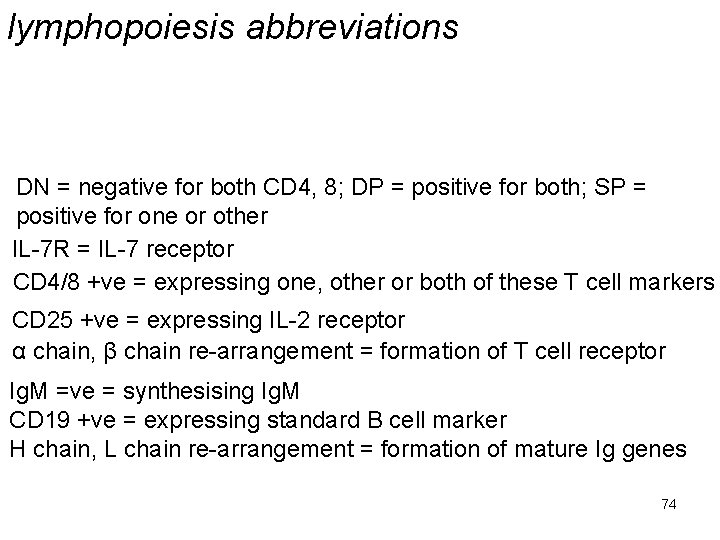
lymphopoiesis abbreviations DN = negative for both CD 4, 8; DP = positive for both; SP = positive for one or other IL-7 R = IL-7 receptor CD 4/8 +ve = expressing one, other or both of these T cell markers CD 25 +ve = expressing IL-2 receptor α chain, β chain re-arrangement = formation of T cell receptor Ig. M =ve = synthesising Ig. M CD 19 +ve = expressing standard B cell marker H chain, L chain re-arrangement = formation of mature Ig genes 74

lymphocytes • The process of lymphopoiesis is driven initially by the growth factor IL-7 – hence the importance of its receptor. • How the different lymphoid lineages are established & what factors influence this is complex and ill-understood – by me at any rate. 75
lymphocytes • Lymphocytes are long lived cells unless they are activated (by cognate antigen). • They then become immune effector cells – most of these will die after a few days by apoptosis – some will become memory cells which are very long lived (decades). 76

Conclusions • Haematopoiesis is a complex business but the outlines are pretty clear. 77
- Slides: 77