Guidelines for Sterile Preparations Seoul National University Bundang

Guidelines for Sterile Preparations Seoul National University Bundang Hospital Hyung Wook Namgung, Rph 031 -787 -3860, 010 -7322 -4180 pinewind@snubh. org
약사에 의한 주사조제의 당위성 Integral part of any health-system Contamination High-alert Medications 관리 Medication Errors 예방 전문분야 집중 잉여약 관리
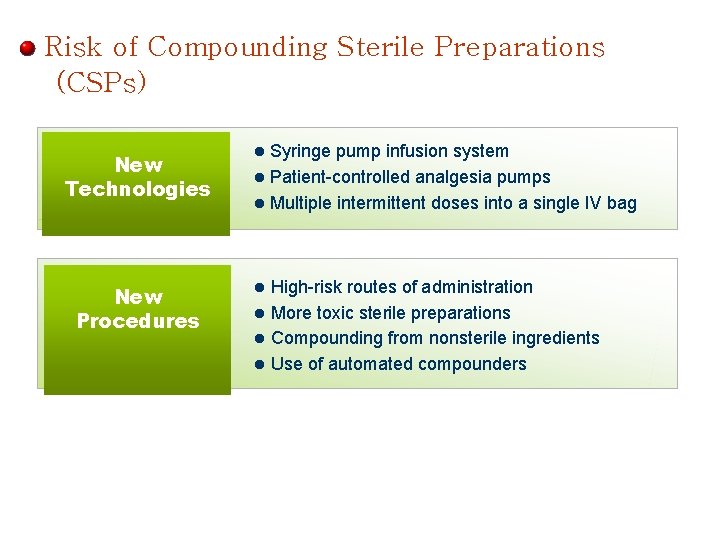
Risk of Compounding Sterile Preparations (CSPs) Syringe pump infusion system l Patient-controlled analgesia pumps l Multiple intermittent doses into a single IV bag New Technologies l New Procedures l High-risk routes of administration l More toxic sterile preparations l Compounding from nonsterile ingredients l Use of automated compounders

Accuracy in Compounding I. V. Products Ref : Am J Health-Syst Pharm. 1997; 54(8): 904 -12
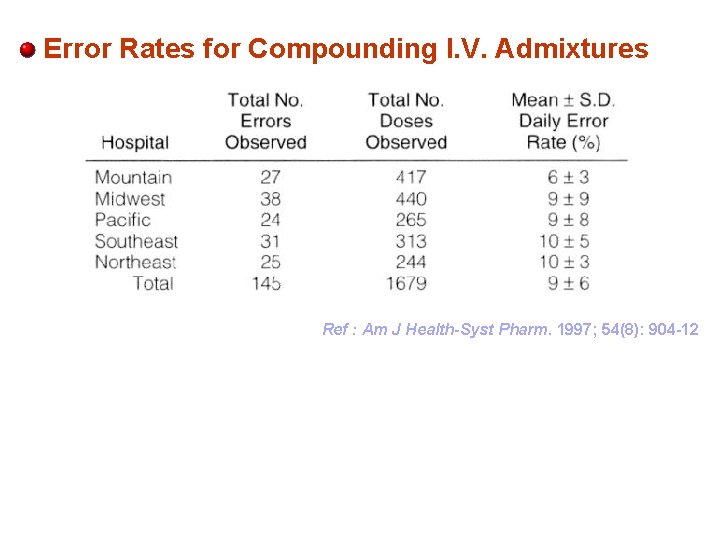
Error Rates for Compounding I. V. Admixtures Ref : Am J Health-Syst Pharm. 1997; 54(8): 904 -12
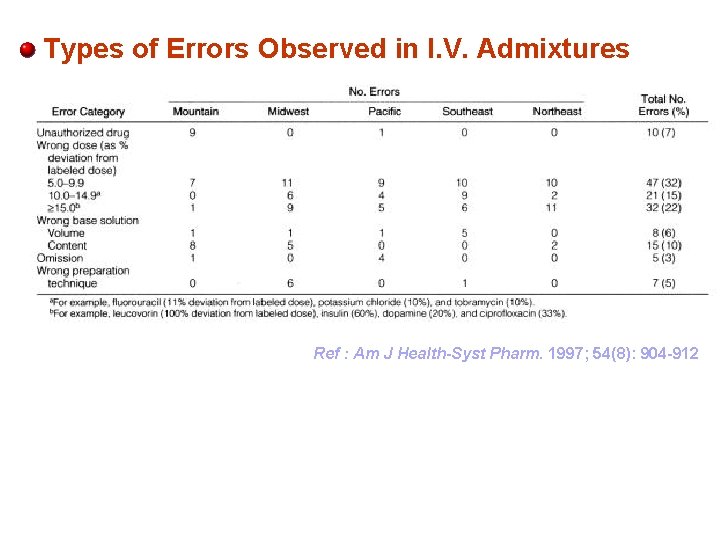
Types of Errors Observed in I. V. Admixtures Ref : Am J Health-Syst Pharm. 1997; 54(8): 904 -912
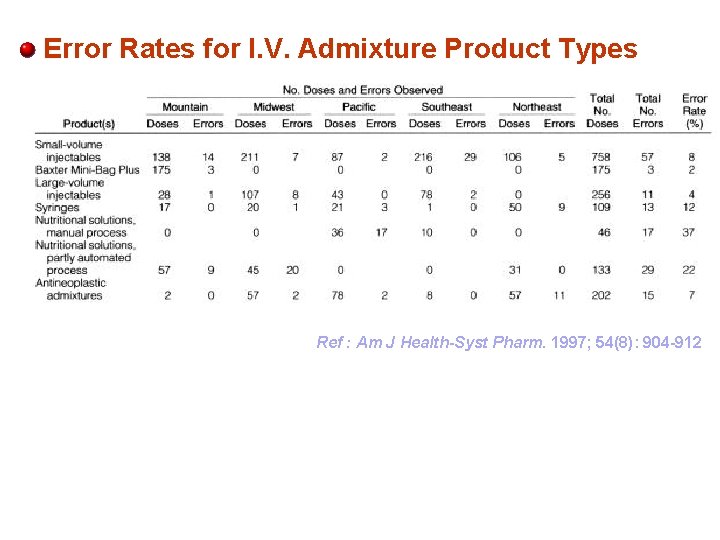
Error Rates for I. V. Admixture Product Types Ref : Am J Health-Syst Pharm. 1997; 54(8): 904 -912
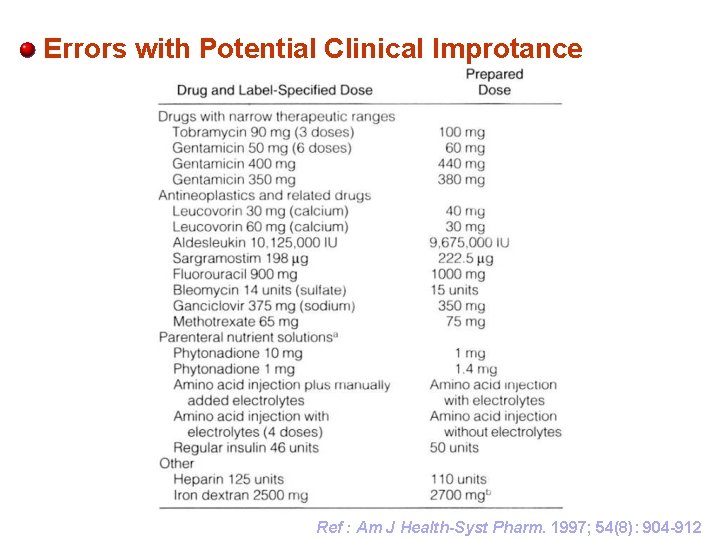
Errors with Potential Clinical Improtance Ref : Am J Health-Syst Pharm. 1997; 54(8): 904 -912

Guideline Sources (I) v The Food and Drug Administration – v The FDA Modernization Act of 1997 (FDAMA) “ Pharmacy Compounding ” The National Association of Boards of Pharmacy (NABP) Model State Pharmacy Practice Act compounding VS manufacturing – Model Rules for Sterile Pharmaceuticals –

Guideline Sources (II) v The United States Pharmacopeia and The National Formulary (USP-NF) and its supplements – v General Information 1206 Sterile drug products for home use => GTA 797 Pharmaceutical compounding – Sterile preparations The American Society of Health-System Pharmacists (ASHP) ASHP technical assistance bulletin on quality assurance for pharmacy-prepared sterile products. Am J Hosp Pharm. 1993; 50(11): 2386 -98 – ASHP guidelines on quality assurance for pharmacy-prepared sterile products. Am J Health Syst Pharm. 2000; 57(12): 1150 -69 – ASHP guidelines on the safe use of automated compounding devices for the preparation of parenteral nutrition admixtures. Am J Health Syst Pharm. 2000; 57(14): 1343 -8 –

Guideline Sources (III) v The American Society for Parenteral and Enteral Nutrition (A. S. P. E. N. ) – Safe practices for parenteral nutrition formulations v The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) v The Centers for Disease Control and Prevention (CDC) v The Occupational Safety and Health Act (OSHA) v International Organization for Standardization (ISO)

Definition of CSPs Preparations prepared according to manufacturer’s labeled instructions v Preparations containing nonsterile ingredients or employing nonsterile components and devices that must be sterilized before administration v Biologics, diagnostics, drugs, nutrients, and radiopharmaceuticals that possess either of the above two characteristics and which include, but are not limited to, baths and soaks for live organs and tissues, implants, inhalations, injections, powder for injection, irrigations, metered sprays and ophthalmic and otic preparations v

Risk Level Classification v potential risk based on – number of exposing patients – Characteristic of ingredients – microbial growth factors influenced by • product storage time • temperature • product ability to support microbial growth • surface and time exposure of critical sites • microbial bioload in the environment

ASHP Risk Level Classification (I) Risk Level 1 1. Products A. Stored at room temperature and completely administered within 28 hours after preparation B. Stored under refrigeration for 7 days or less before complete administration to a patient over a period not to exceed 24 hours C. Frozen for 30 days or less before complete administration to a patient over a period not to exceed 24 hours.

ASHP Risk Level Classification (II) (continued) 2. Unpreserved sterile products prepared for administration to one patient or batch-prepared products containing suitable preservatives prepared for administration to more than one patient. 3. Products prepared by closed-system aseptic transfer of sterile, nonpyrogenic, finished pharmaceuticals (e. g. , from vials or ampuls) obtained from licensed manufacturers into sterile final containers (e. g. , syringes, minibags, elastomeric containers, portable infusiondevice cassettes) obtained from licensed manufacturers.

ASHP Risk Level Classification (III) Risk Level 2 1. Products stored beyond 7 days under refrigeration, stored beyond 30 days frozen, or administered beyond 28 hours after preparation and storage at room temperature 2. Batch-prepared products without preservatives that are intended for use by more than one patient. 3. Products compounded by complex or numerous manipulations of sterile ingredients obtained from licensed manufacturers in a sterile container or reservoir obtained from a licensed manufacturer by using closed-system aseptic transfer

ASHP Risk Level Classification (IV) Risk Level 3 1. Products compounded from nonsterile ingredients or compounded with nonsterile components, containers, or equipment before terminal sterilization. 2. Products prepared by combining multiple ingredients-sterile or non sterile-by using an open-system transfer or open reservoir before terminal sterilization.

USP 797 Risk Level Classification (I) Low-Risk Level v CSPs compounded from sterile commercial drugs using commercial sterile devices v Compounding occurs in ISO Class 5 environment at all times v Compounding procedures involves only a few closed system basic, simple aseptic transfers and manipulations v Routine disinfection and air quality testing to maintain ISO Class 5 occurs v There is adequate personnel garb for sterile preparation v A review for correct identity and amounts of components occurs before and after compounding v There is a final visual inspection of each sterile preparation v Annual media-fill test procedure for each person who compounds is performed to validate proper aseptic technique

USP 797 Risk Level Classification (II) Medium-Risk Level v Involves using multiple pooled sterile commercial products for multiple patients or one patient multiple times (batched antibiotics or other small volume parenterals) v Involves complex aseptic manipulations (TPN or other multipleingredient CSPs) v Compounding occurs over a prolonged period of time (complex procedures) v No bacteriostatic agents are added to the preparation and it is administered over several days (chemotherapy or pain management administered via infusion device) v Quality assurance procedures include all steps for low-risk level v Requires more challenging annual media-fill evaluation of compounding personnel technique that simulates the most challenging or stressful conditions

USP Risk Level Classification (III) High-Risk Level v Prepared from non-sterile ingredients v Preparation from sterile ingredients but exposed to less than ISO Class 5 v More than 6 -hour delay from compounding to sterilization v Purity of components is assumed but not verified by documentation v Quality assurance procedures include all steps for low-risk level v Requires a semiannual media-fill evaluation of compounding personnel technique that simulates the most challenging or stressful conditions using dry nonsterile media verification of compounding personnel technique v Requires simulation of each high-risk level compounding sterilization process using dry nonsterile media verification
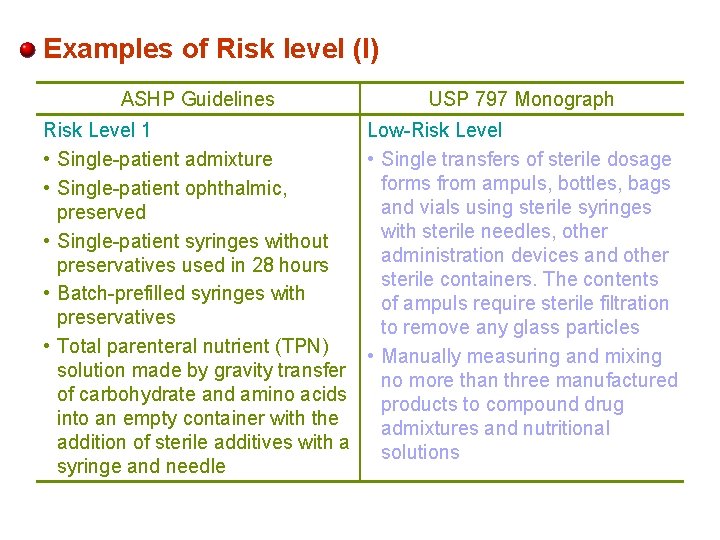
Examples of Risk level (I) ASHP Guidelines USP 797 Monograph Risk Level 1 Low-Risk Level • Single-patient admixture • Single transfers of sterile dosage forms from ampuls, bottles, bags • Single-patient ophthalmic, and vials using sterile syringes preserved with sterile needles, other • Single-patient syringes without administration devices and other preservatives used in 28 hours sterile containers. The contents • Batch-prefilled syringes with of ampuls require sterile filtration preservatives to remove any glass particles • Total parenteral nutrient (TPN) • Manually measuring and mixing solution made by gravity transfer no more than three manufactured of carbohydrate and amino acids products to compound drug into an empty container with the admixtures and nutritional addition of sterile additives with a solutions syringe and needle
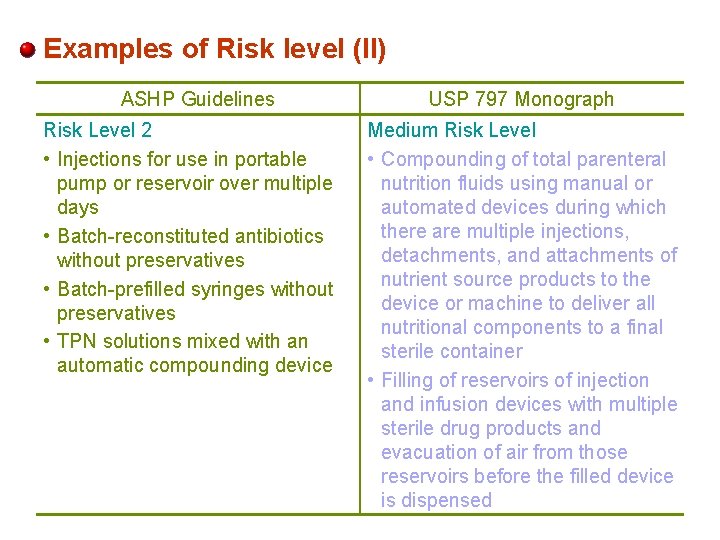
Examples of Risk level (II) ASHP Guidelines Risk Level 2 • Injections for use in portable pump or reservoir over multiple days • Batch-reconstituted antibiotics without preservatives • Batch-prefilled syringes without preservatives • TPN solutions mixed with an automatic compounding device USP 797 Monograph Medium Risk Level • Compounding of total parenteral nutrition fluids using manual or automated devices during which there are multiple injections, detachments, and attachments of nutrient source products to the device or machine to deliver all nutritional components to a final sterile container • Filling of reservoirs of injection and infusion devices with multiple sterile drug products and evacuation of air from those reservoirs before the filled device is dispensed
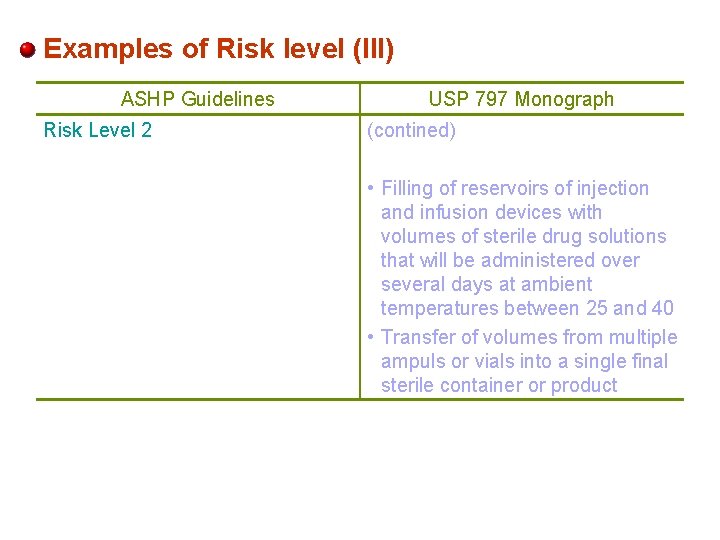
Examples of Risk level (III) ASHP Guidelines Risk Level 2 USP 797 Monograph (contined) • Filling of reservoirs of injection and infusion devices with volumes of sterile drug solutions that will be administered over several days at ambient temperatures between 25 and 40 • Transfer of volumes from multiple ampuls or vials into a single final sterile container or product
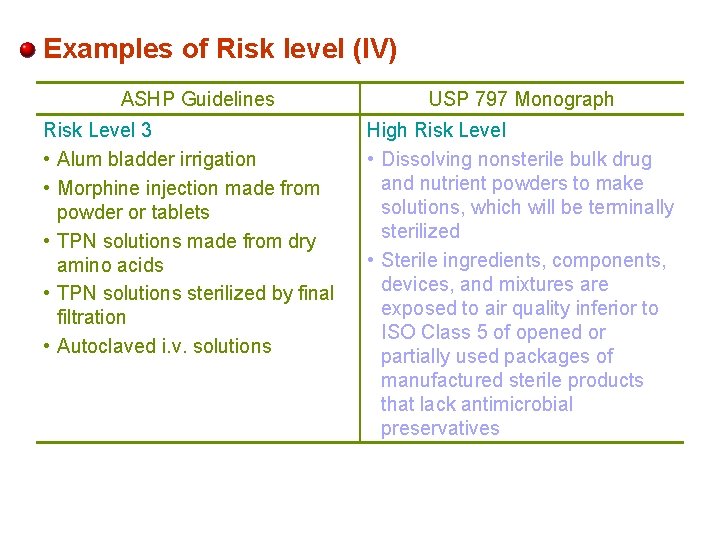
Examples of Risk level (IV) ASHP Guidelines Risk Level 3 • Alum bladder irrigation • Morphine injection made from powder or tablets • TPN solutions made from dry amino acids • TPN solutions sterilized by final filtration • Autoclaved i. v. solutions USP 797 Monograph High Risk Level • Dissolving nonsterile bulk drug and nutrient powders to make solutions, which will be terminally sterilized • Sterile ingredients, components, devices, and mixtures are exposed to air quality inferior to ISO Class 5 of opened or partially used packages of manufactured sterile products that lack antimicrobial preservatives
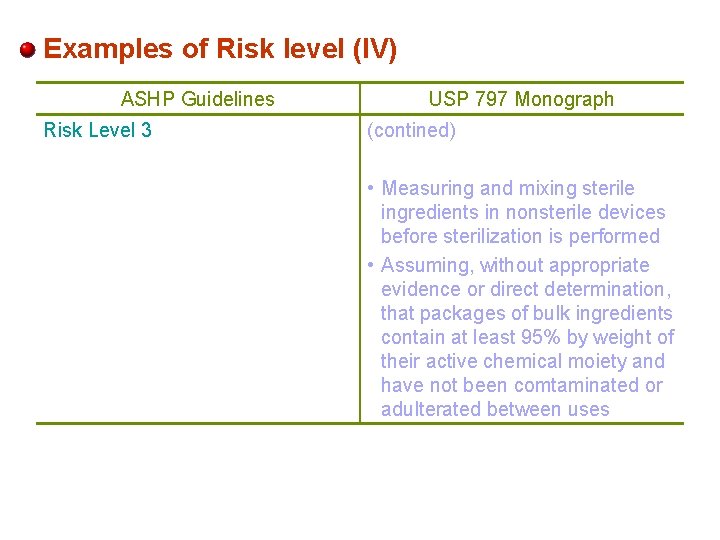
Examples of Risk level (IV) ASHP Guidelines Risk Level 3 USP 797 Monograph (contined) • Measuring and mixing sterile ingredients in nonsterile devices before sterilization is performed • Assuming, without appropriate evidence or direct determination, that packages of bulk ingredients contain at least 95% by weight of their active chemical moiety and have not been comtaminated or adulterated between uses
- Slides: 25