Guide Questions Why is the periodic table so
Guide Questions Why is the periodic table so important? Why is the periodic table shaped the way it's shaped? Why do elements combine? Why do elements react? What other patterns are there in the world and how do they help us?
THE DEVELOPMENT OF THE PERIODIC TABLE
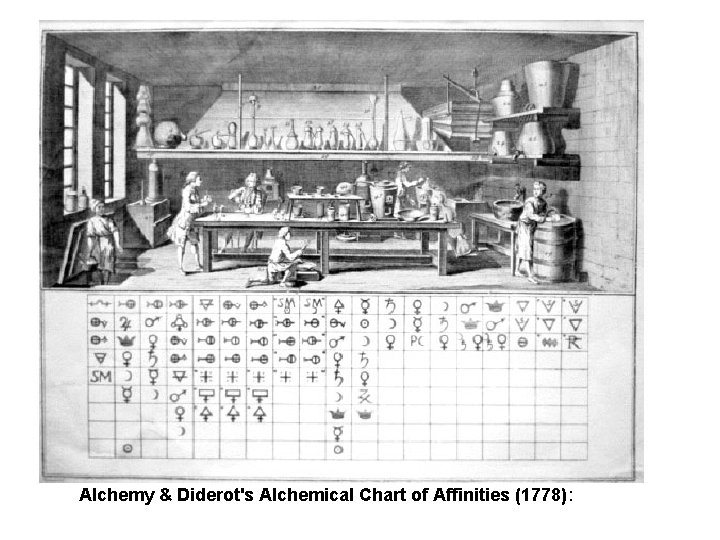
Alchemy & Diderot's Alchemical Chart of Affinities (1778):
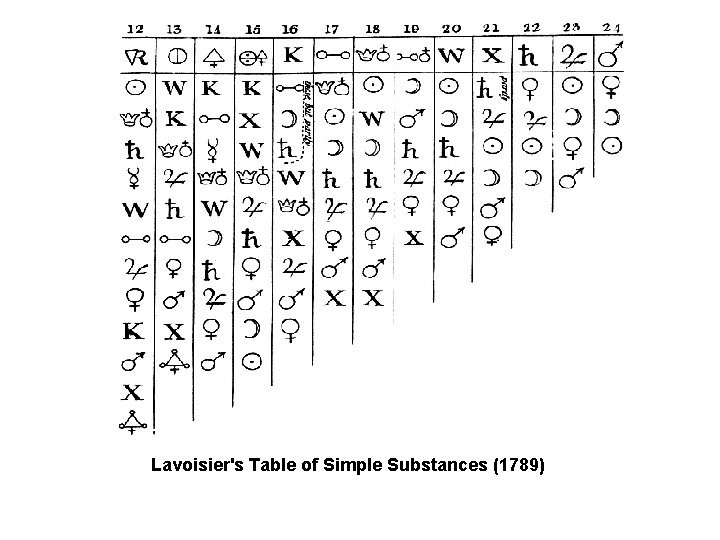
Lavoisier's Table of Simple Substances (1789)
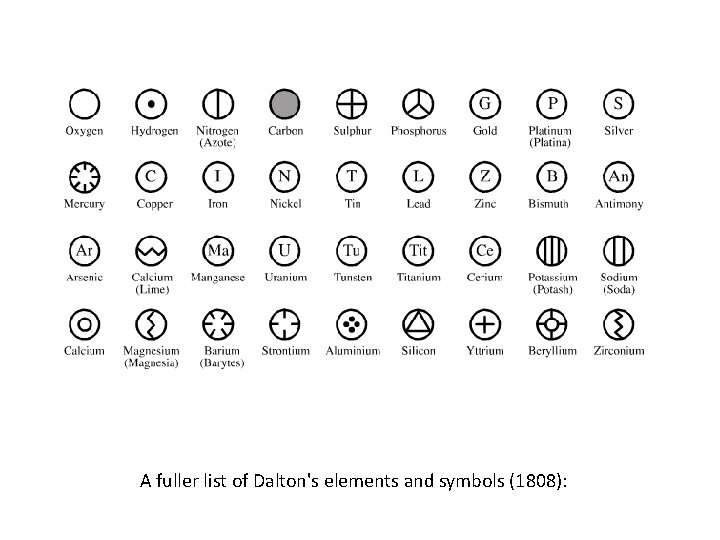
A fuller list of Dalton's elements and symbols (1808):
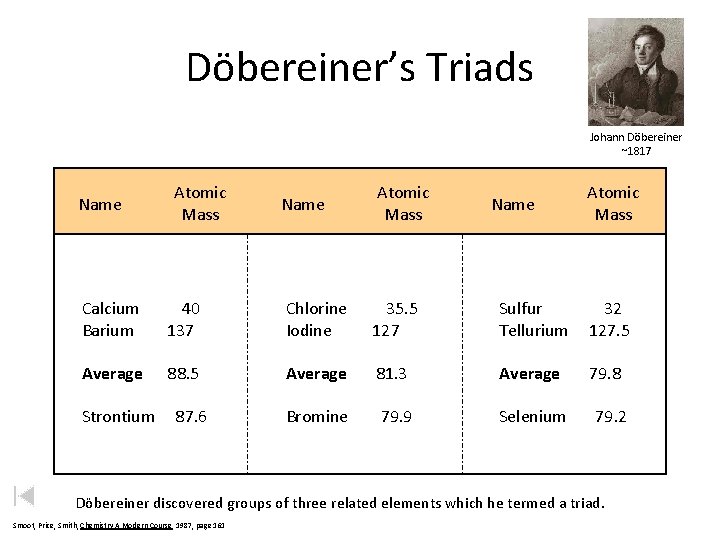
Döbereiner’s Triads Johann Döbereiner ~1817 Name Atomic Mass Calcium Barium 40 137 Chlorine Iodine 35. 5 127 Sulfur Tellurium 32 127. 5 Average 88. 5 Average 81. 3 Average 79. 8 Bromine 79. 9 Selenium Strontium 87. 6 79. 2 Döbereiner discovered groups of three related elements which he termed a triad. Smoot, Price, Smith, Chemistry A Modern Course 1987, page 161
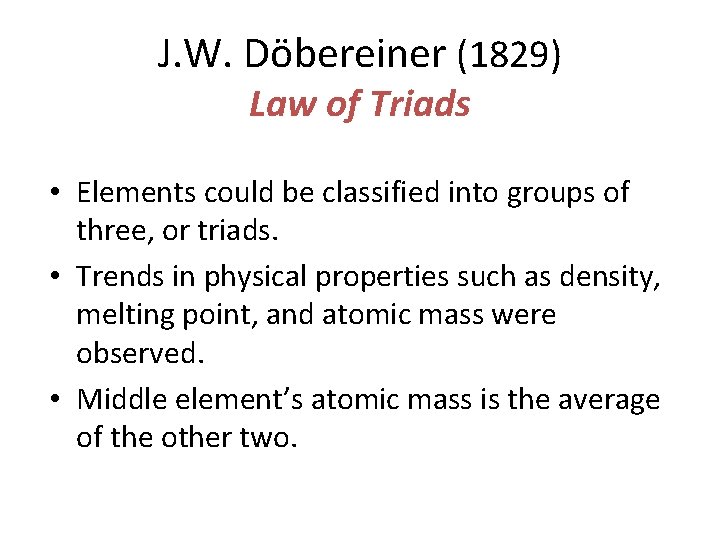
J. W. Döbereiner (1829) Law of Triads • Elements could be classified into groups of three, or triads. • Trends in physical properties such as density, melting point, and atomic mass were observed. • Middle element’s atomic mass is the average of the other two.

The Telluric Helix or Screw (1862)
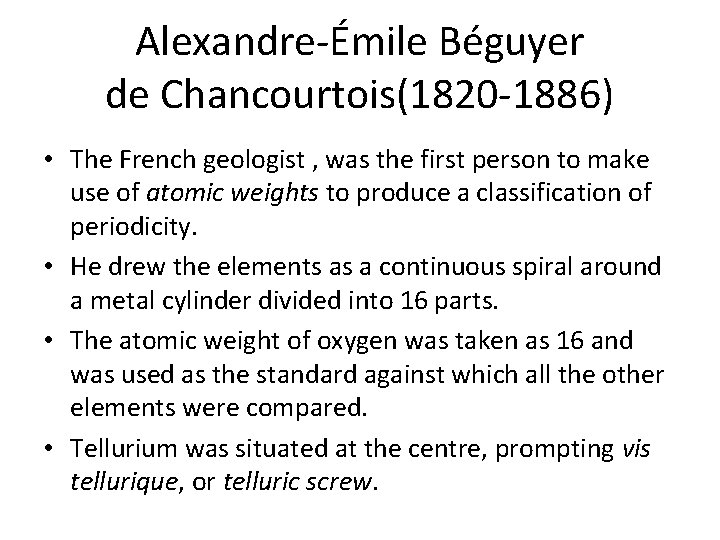
Alexandre-Émile Béguyer de Chancourtois(1820 -1886) • The French geologist , was the first person to make use of atomic weights to produce a classification of periodicity. • He drew the elements as a continuous spiral around a metal cylinder divided into 16 parts. • The atomic weight of oxygen was taken as 16 and was used as the standard against which all the other elements were compared. • Tellurium was situated at the centre, prompting vis tellurique, or telluric screw.
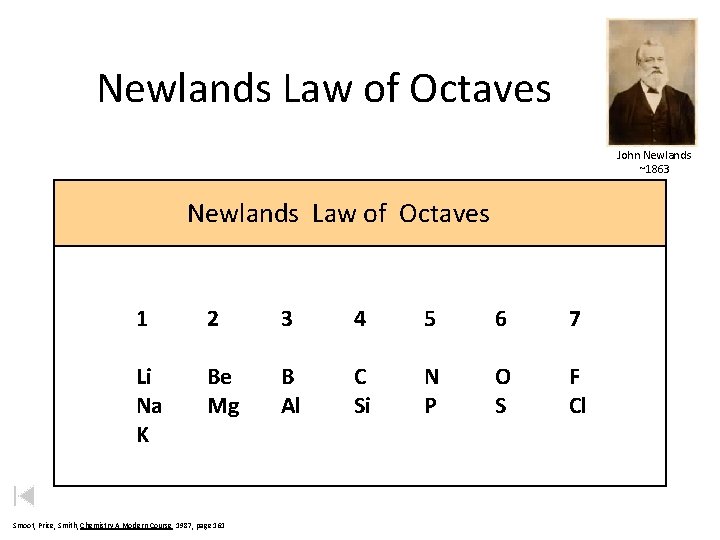
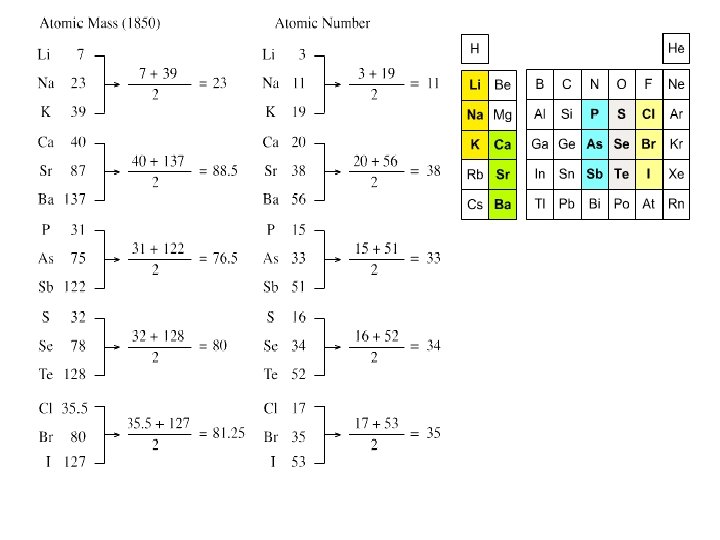
Newlands Law of Octaves John Newlands ~1863 Newlands Law of Octaves 1 2 3 4 5 6 7 Li Na K Be Mg B Al C Si N P O S F Cl Smoot, Price, Smith, Chemistry A Modern Course 1987, page 161
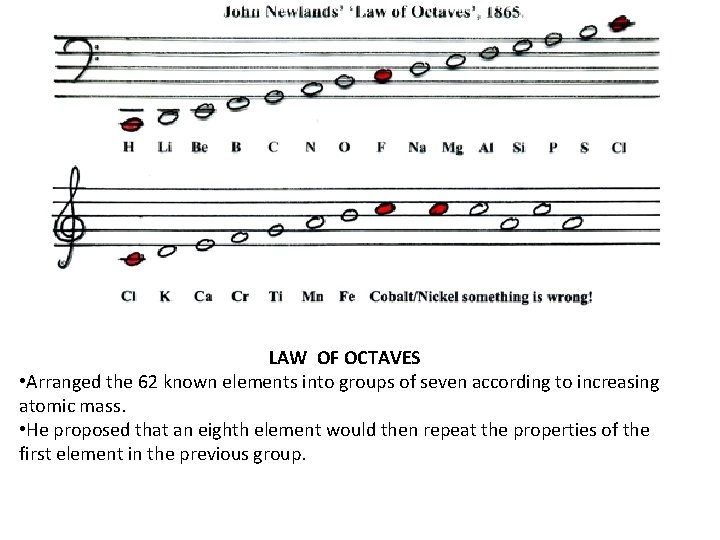
TAVES LAW OF OCTAVES • Arranged the 62 known elements into groups of seven according to increasing atomic mass. • He proposed that an eighth element would then repeat the properties of the first element in the previous group.

Dmitri Ivanovich Mendeleev • Russian • Invented periodic table • In 1860’s proposed new arrangements of elements by properties • Arranged elements by atomic mass • Left gaps in the Periodic table for future elements yet discovered • Predicted existence of several unknown elements • Element 101 Dmitri Mendeleev

• In the 1860’s, Mendeleev and the German chemist Lothar Meyer, each working alone, made an eight-column table of the elements. • However, Mendeleev had to leave some blank spots in order to group all the elements with similar properties in the same column. To explain these blank spots, Mendeleev

• . On the basis of his arrangement, Mendeleev predicted the properties and atomic masses of several elements that were unknown at the time. • Mendeleev left blanks in his table for undiscovered elements. • Mendeleev predicted properties and masses of unknown elements correctly.
Dmitri Mendeléev
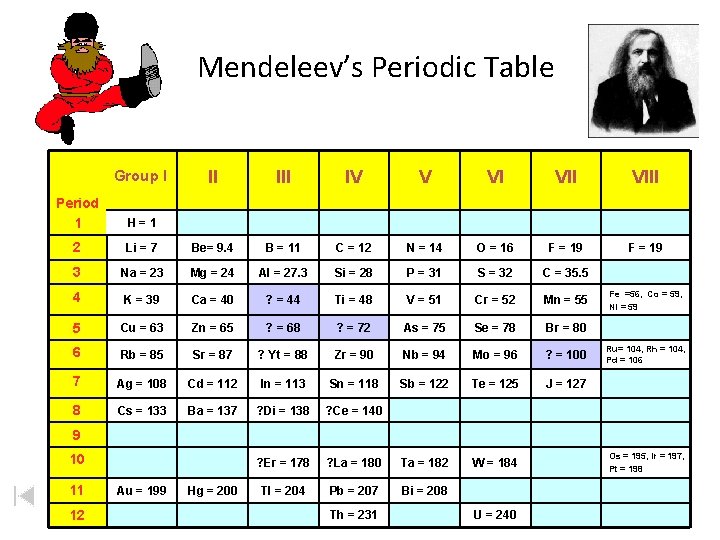
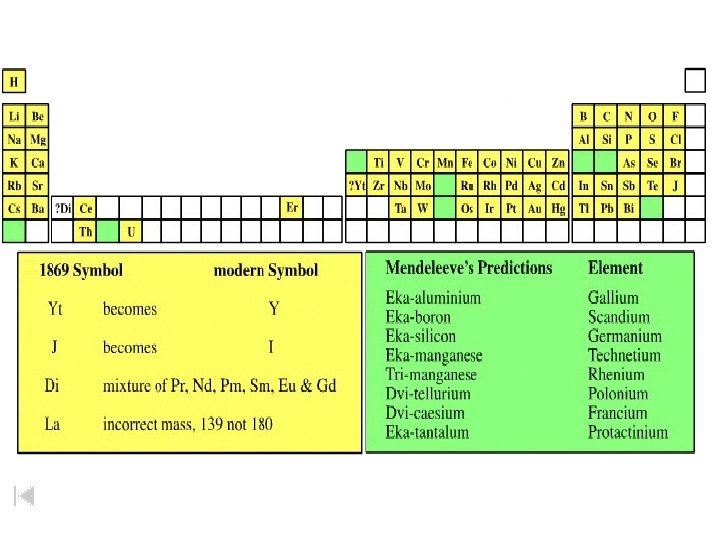
Mendeleev’s Periodic Table Group I II IV V VI VIII F = 19 Period 1 H=1 2 Li = 7 Be= 9. 4 B = 11 C = 12 N = 14 O = 16 F = 19 3 Na = 23 Mg = 24 Al = 27. 3 Si = 28 P = 31 S = 32 C = 35. 5 4 K = 39 Ca = 40 ? = 44 Ti = 48 V = 51 Cr = 52 Mn = 55 5 Cu = 63 Zn = 65 ? = 68 ? = 72 As = 75 Se = 78 Br = 80 6 Rb = 85 Sr = 87 ? Yt = 88 Zr = 90 Nb = 94 Mo = 96 ? = 100 7 Ag = 108 Cd = 112 In = 113 Sn = 118 Sb = 122 Te = 125 J = 127 8 Cs = 133 Ba = 137 ? Di = 138 ? Ce = 140 ? Er = 178 ? La = 180 Ta = 182 W = 184 Tl = 204 Pb = 207 Bi = 208 Fe =56, Co = 59, Ni = 59 Ru= 104, Rh = 104, Pd = 106 9 10 11 12 Au = 199 Hg = 200 Th = 231 U = 240 Os = 195, Ir = 197, Pt = 198
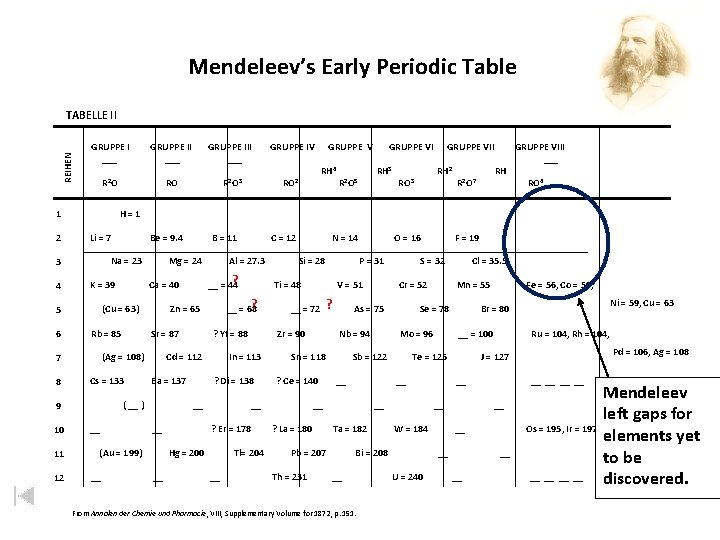
Mendeleev’s Early Periodic Table REIHEN TABELLE II GRUPPE I ___ Li = 7 11 12 GRUPPE V RH 4 Sr = 87 Cs = 133 GRUPPE VI RH 3 R 2 O 5 ? Yt = 88 Ba = 137 ? Di = 138 __ (Au = 199) GRUPPE VII RH 2 RO 3 ? Er = 178 Tl= 204 __ O = 16 P = 31 V = 51 __ = 72 ? RH R 2 O 7 GRUPPE VIII ___ RO 4 ? La = 180 __ = 100 __ Ta = 182 __ From Annalen der Chemie und Pharmacie, Pharmacie VIII, Supplementary Volume for 1872, p. 151. Pd = 106, Ag = 108 __ __ U = 240 Ru = 104, Rh = 104, __ W = 184 Ni = 59, Cu = 63 J = 127 __ Bi = 208 Fe = 56, Co = 59, Br = 80 Te = 125 __ Pb = 207 Mn = 55 Mo = 96 __ Cl = 35. 5 Se = 78 Sb = 122 __ Th = 231 Cr = 52 Nb = 94 ? Ce = 140 F = 19 S = 32 As = 75 Sn = 118 __ Hg = 200 __ Si = 28 Zr = 90 In = 113 __ N = 14 Ti = 48 ? __ = 68 Cd = 112 ( __ ) __ Al = 27. 3 Zn = 65 (Ag = 108) C = 12 ? __ = 44 Ca = 40 Rb = 85 __ B = 11 Mg = 24 (Cu = 63) 9 10 RO 2 Be = 9. 4 K = 39 7 8 RO R 2 O 3 Na = 23 5 6 GRUPPE IV H=1 3 4 GRUPPE III ___ R 2 O 1 2 GRUPPE II ___ __ __ Mendeleev left gaps for Os = 195, Ir = 197, elements yet Pt = 198, Au = 199 to be __ __ discovered.
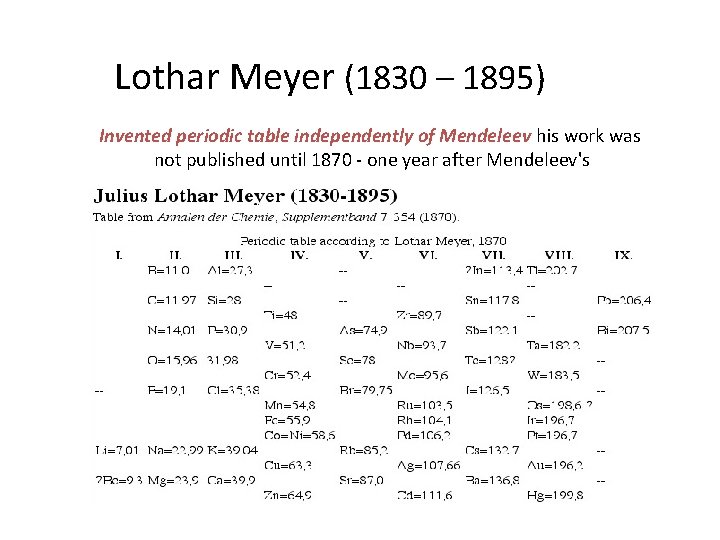
Lothar Meyer (1830 – 1895) Invented periodic table independently of Mendeleev his work was not published until 1870 - one year after Mendeleev's
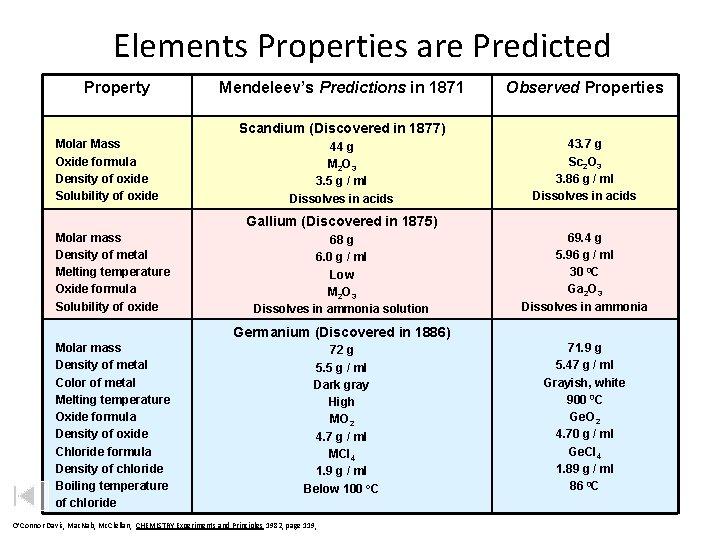
Elements Properties are Predicted Property Mendeleev’s Predictions in 1871 Observed Properties Scandium (Discovered in 1877) Molar Mass Oxide formula Density of oxide Solubility of oxide 44 g M 2 O 3 3. 5 g / ml Dissolves in acids 43. 7 g Sc 2 O 3 3. 86 g / ml Dissolves in acids Gallium (Discovered in 1875) Molar mass Density of metal Melting temperature Oxide formula Solubility of oxide 68 g 6. 0 g / ml Low M 2 O 3 Dissolves in ammonia solution 69. 4 g 5. 96 g / ml 30 0 C Ga 2 O 3 Dissolves in ammonia Germanium (Discovered in 1886) Molar mass Density of metal Color of metal Melting temperature Oxide formula Density of oxide Chloride formula Density of chloride Boiling temperature of chloride 72 g 5. 5 g / ml Dark gray High MO 2 4. 7 g / ml MCl 4 1. 9 g / ml Below 100 o. C O’Connor Davis, Mac. Nab, Mc. Clellan, CHEMISTRY Experiments and Principles 1982, page 119, 71. 9 g 5. 47 g / ml Grayish, white 900 0 C Ge. O 2 4. 70 g / ml Ge. Cl 4 1. 89 g / ml 86 0 C

Modern Periodic Law Henry Moseley (1887 -1915) • subjected known elements to x-rays and was able to derive a relationship between x-ray frequency and number of protons. • arranged the elements according to increasing atomic numbers and not atomic masses, some of the inconsistencies associated with Mendeleev's table were eliminated. • The modern periodic table is based on Moseley's Periodic Law (atomic numbers).
Extended periodic table

Other Periodic tables
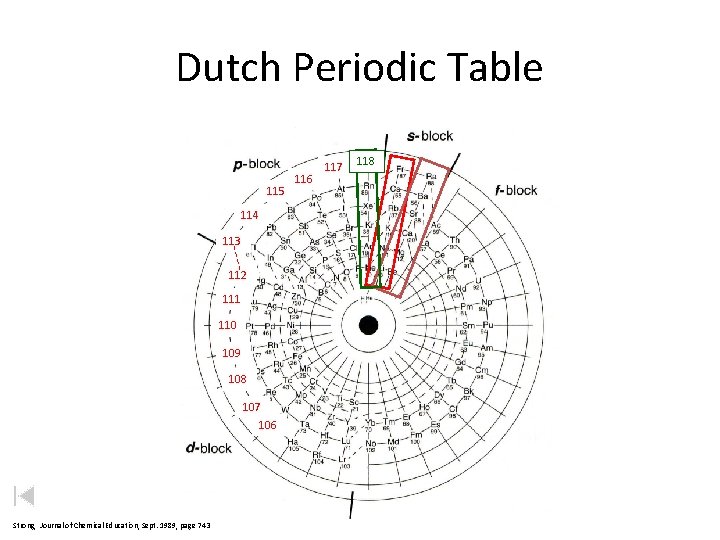
Dutch Periodic Table 115 114 113 112 111 110 109 108 107 106 Strong, Journal of Chemical Education, Sept. 1989, page 743 116 117 118
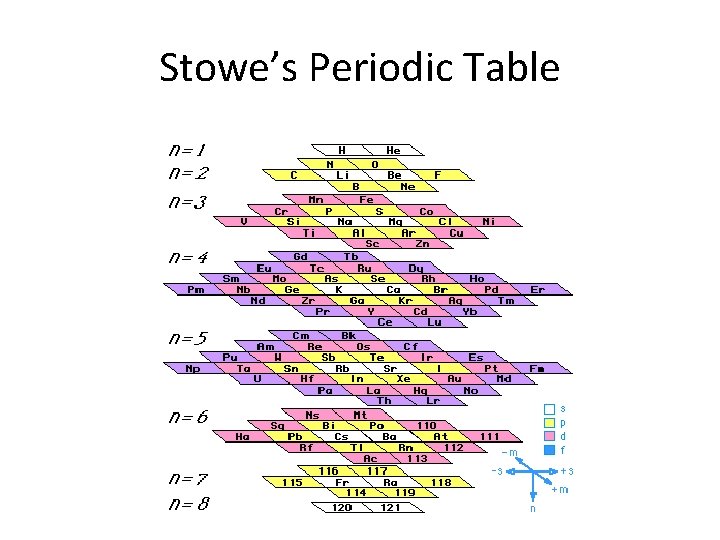
Stowe’s Periodic Table
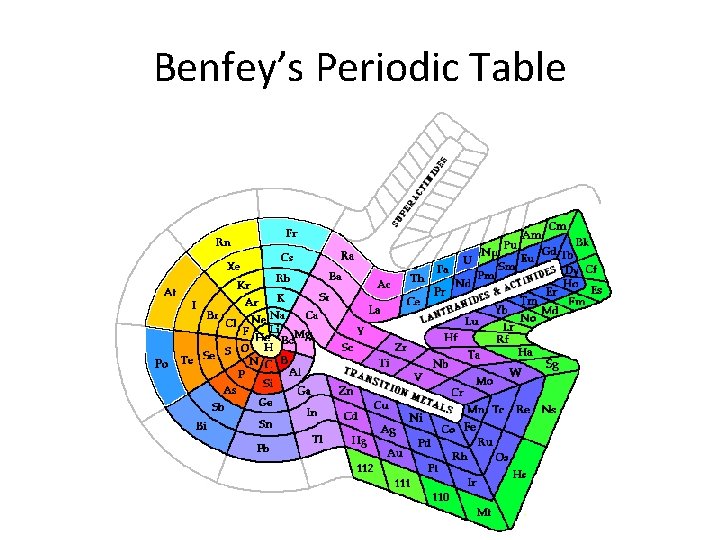
Benfey’s Periodic Table
ELECTRON CONFIGURATION AND THE PERIODIC TABLE
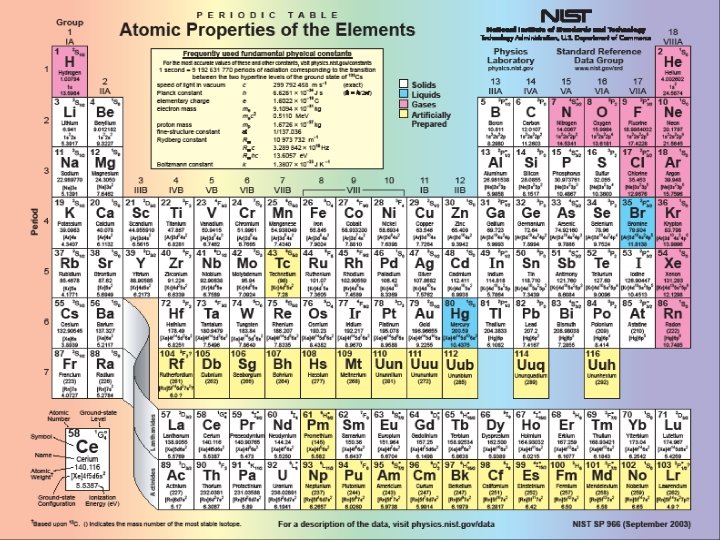
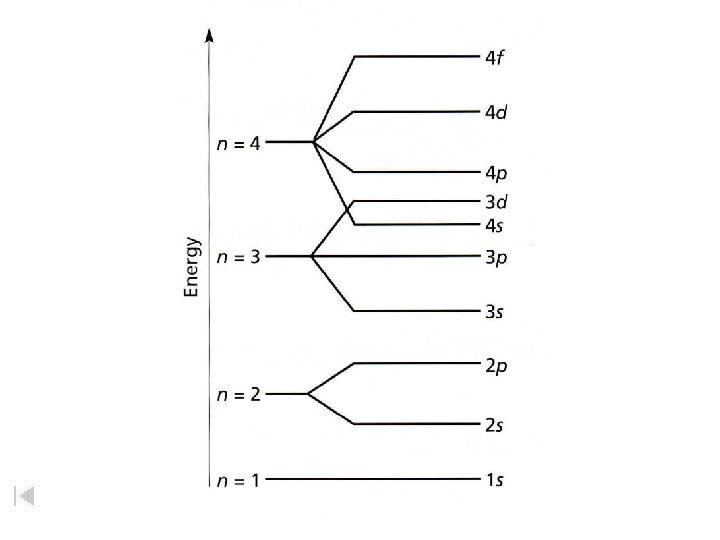
GROUP - vertical columns in the periodic table PERIOD - horizontal rows in the periodic table BLOCK - 4 areas in the periodic table representing the orbital type
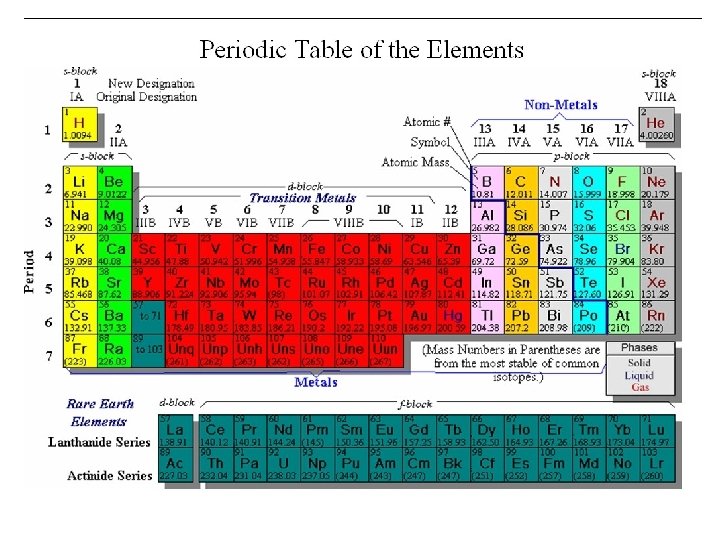
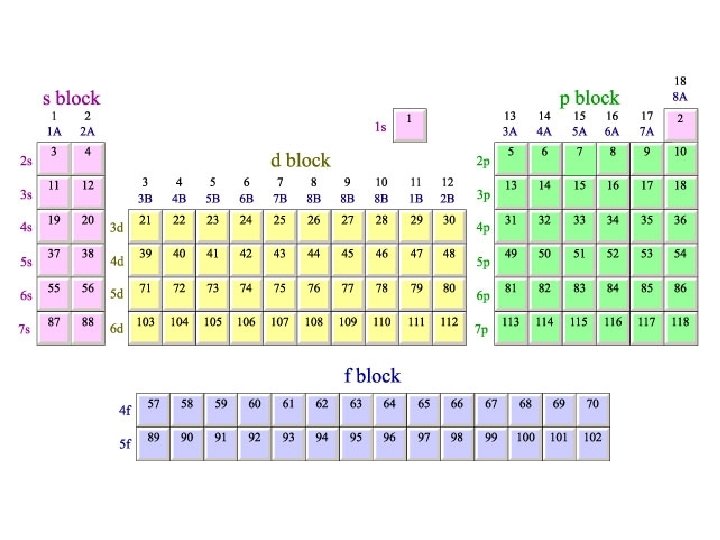
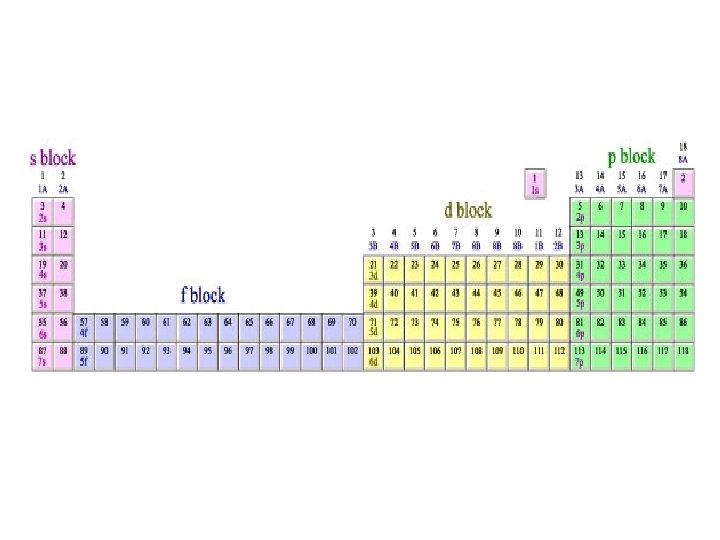
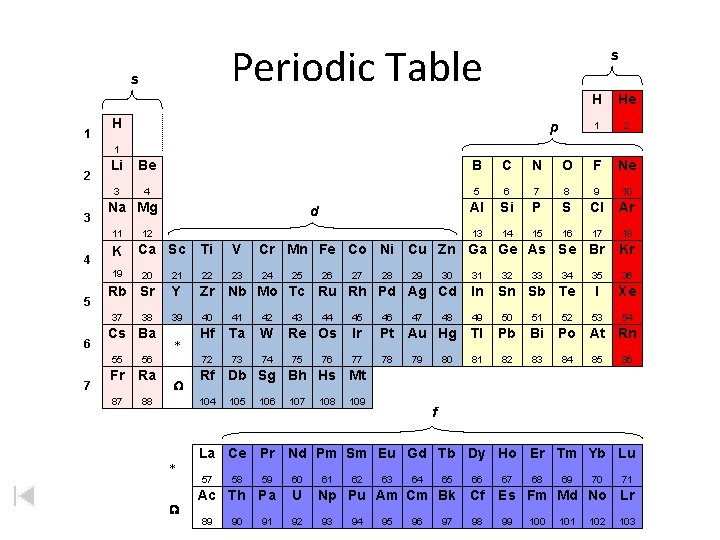
Periodic Table s 1 s H p H He 1 2 3 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Na Mg 11 4 K 19 5 7 12 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 d 38 Cs Ba 55 56 Fr Ra 87 88 * W 44 Re Os 75 76 27 28 29 30 47 48 31 32 33 Sn Sb Te 45 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 79 80 34 52 Po At Rn 84 85 86 105 106 107 108 109 f La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 W 43 26 Rf Db Sg Bh Hs Mt 104 * 25 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103
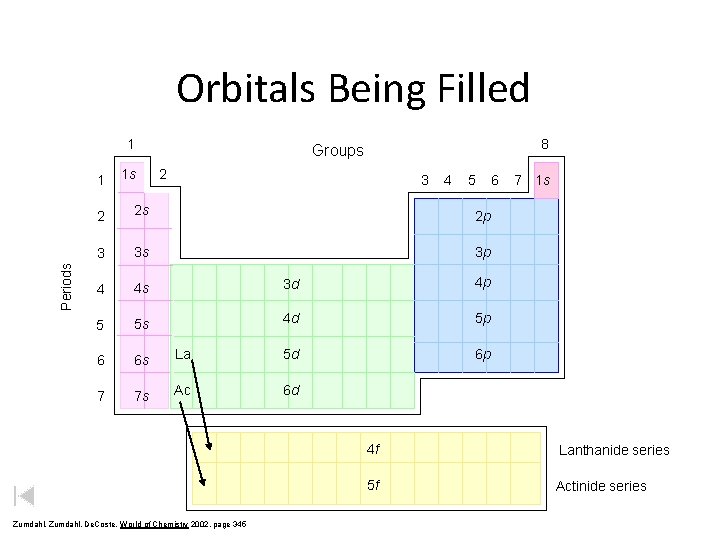
Orbitals Being Filled 1 Periods 1 1 s 8 Groups 2 3 4 5 2 2 s 2 p 3 3 s 3 p 4 4 s 3 d 4 p 5 5 s 4 d 5 p 6 6 s La 5 d 6 p 7 7 s Ac 6 d Zumdahl, De. Coste, World of Chemistry 2002, page 345 6 7 1 s 4 f Lanthanide series 5 f Actinide series
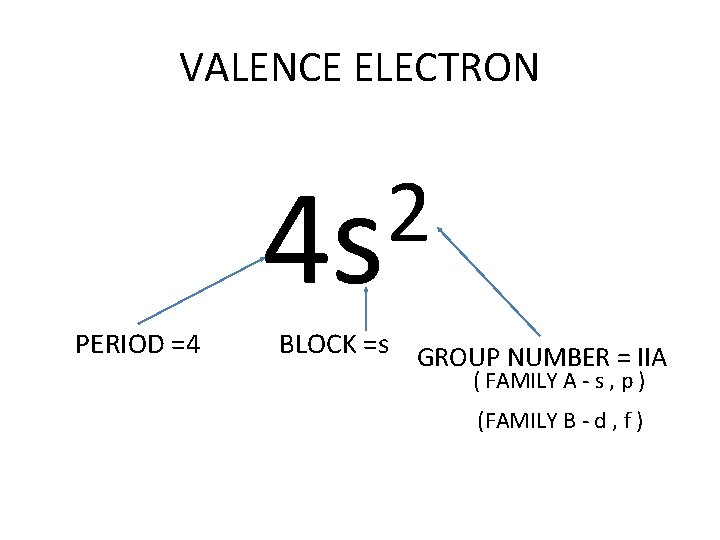
VALENCE ELECTRON 2 4 s PERIOD =4 BLOCK =s GROUP NUMBER = IIA ( FAMILY A - s , p ) (FAMILY B - d , f )
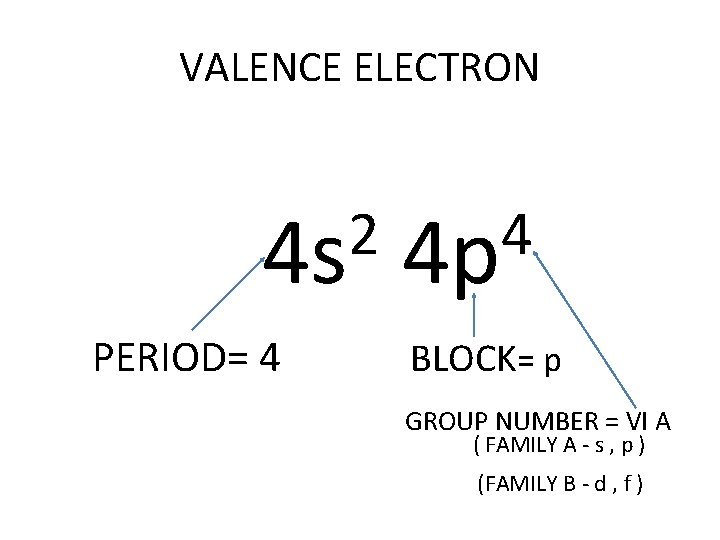
VALENCE ELECTRON 2 4 s PERIOD= 4 4 4 p BLOCK= p GROUP NUMBER = VI A ( FAMILY A - s , p ) (FAMILY B - d , f )
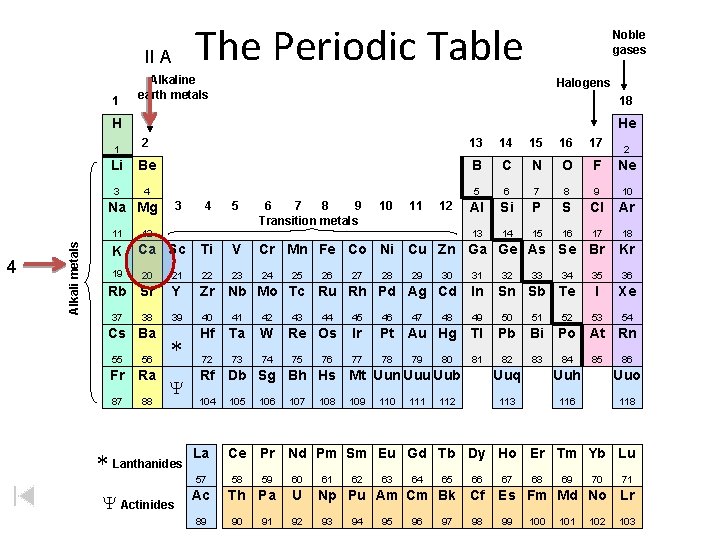
The Periodic Table II A 1 Noble gases Alkaline earth metals Halogens 18 H He 2 13 14 15 16 17 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 1 3 Na Mg 4 Alkali metals 11 K 19 4 5 6 7 8 9 Transition metals 10 11 12 12 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 * Hf Ta W 72 73 74 37 38 Cs Ba 55 56 Fr Ra 87 88 Y * Lanthanides 25 43 26 44 Re Os 75 76 27 28 29 47 30 104 La Ac 89 105 106 107 108 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 109 110 111 112 34 Sn Sb Te 45 79 48 31 Rf Db Sg Bh Hs Mt Uun Uuu Uub 57 Y Actinides 2 52 Po At Rn 84 85 86 Uuq Uuh Uuo 113 116 118 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 58 59 60 Th Pa U 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103
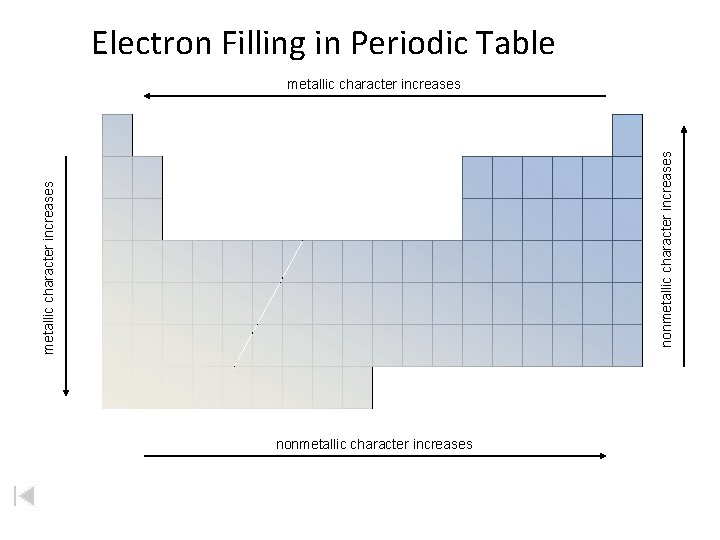
Electron Filling in Periodic Table metallic character increases nonmetallic character increases
1 H H He 1 2 3 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Al Si P S Cl Ar 13 14 15 16 17 18 Na Mg 11 4 K 19 5 7 Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 35 36 I Xe 53 54 20 21 22 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 39 40 41 42 49 50 51 Hf Ta W 72 73 74 37 6 12 38 Cs Ba 55 56 Fr Ra 87 88 * W 25 43 26 44 Re Os 75 76 27 28 29 47 30 32 33 46 Ir Pt Au Hg Tl Pb Bi 77 78 81 82 83 80 34 Sn Sb Te 45 79 48 31 52 Po At Rn 84 85 86 Rf Db Sg Bh Hs Mt 104 105 106 107 108 109 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 57 59 60 Ac Th Pa U 89 58 90 91 92 61 62 63 64 65 66 Np Pu Am Cm Bk Cf 93 94 95 96 97 98 67 68 69 70 71 Es Fm Md No Lr 99 100 101 102 103
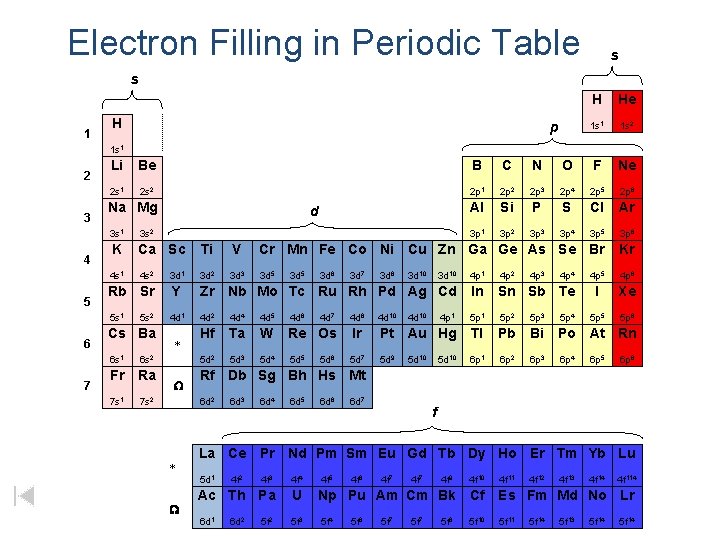
Electron Filling in Periodic Table s s 1 H p H He 1 s 1 1 s 2 1 s 1 2 3 4 5 6 7 Li Be B C N O F Ne 2 s 1 2 s 2 2 p 1 2 p 2 2 p 3 2 p 4 2 p 5 2 p 6 Al Si P S Cl Ar 3 p 1 3 p 2 3 p 3 3 p 4 3 p 5 3 p 6 Na Mg d 3 s 1 3 s 2 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 4 s 1 4 s 2 3 d 1 3 d 2 3 d 3 3 d 5 3 d 10 4 p 1 4 p 2 4 p 5 4 p 6 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 5 s 1 4 d 2 4 d 4 4 d 5 4 d 6 4 d 7 4 d 8 4 d 10 4 p 1 5 p 2 5 p 3 5 p 4 5 p 5 5 p 6 Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 5 d 2 5 d 3 5 d 4 5 d 5 5 d 7 5 d 9 6 p 1 6 p 2 6 p 3 6 p 4 5 s 2 Cs Ba 6 s 1 6 s 2 Fr Ra 7 s 1 7 s 2 * W 5 d 6 3 d 7 3 d 8 3 d 10 4 d 10 5 d 10 4 p 3 4 p 4 6 p 5 6 p 6 6 d 3 6 d 4 6 d 5 6 d 6 6 d 7 f La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 5 d 1 W 3 d 6 Rf Db Sg Bh Hs Mt 6 d 2 * 3 d 5 4 f 2 4 f 3 4 f 4 Ac Th Pa U 6 d 1 5 f 3 6 d 2 5 f 2 4 f 5 4 f 6 4 f 7 4 f 9 4 f 10 Np Pu Am Cm Bk Cf 5 f 4 5 f 6 5 f 7 5 f 8 5 f 10 4 f 11 4 f 12 4 f 13 4 f 14 4 f 114 Es Fm Md No Lr 5 f 11 5 f 14 5 f 13 5 f 14
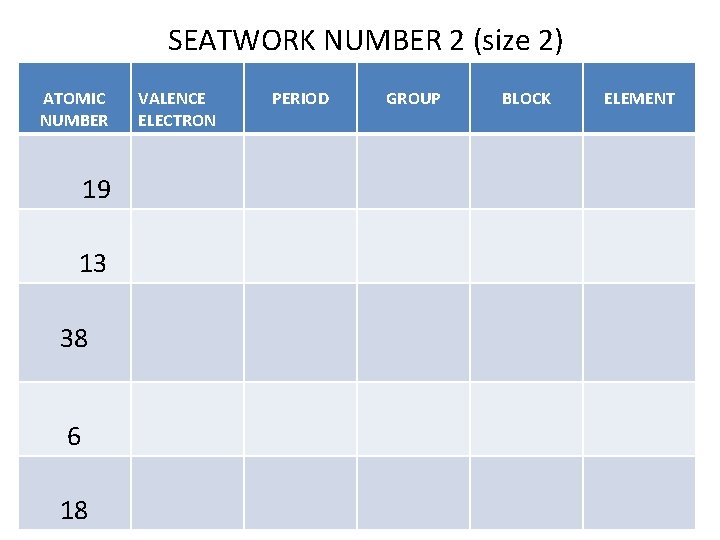
SEATWORK NUMBER 2 (size 2) ATOMIC NUMBER 19 13 38 6 18 VALENCE ELECTRON PERIOD GROUP BLOCK ELEMENT
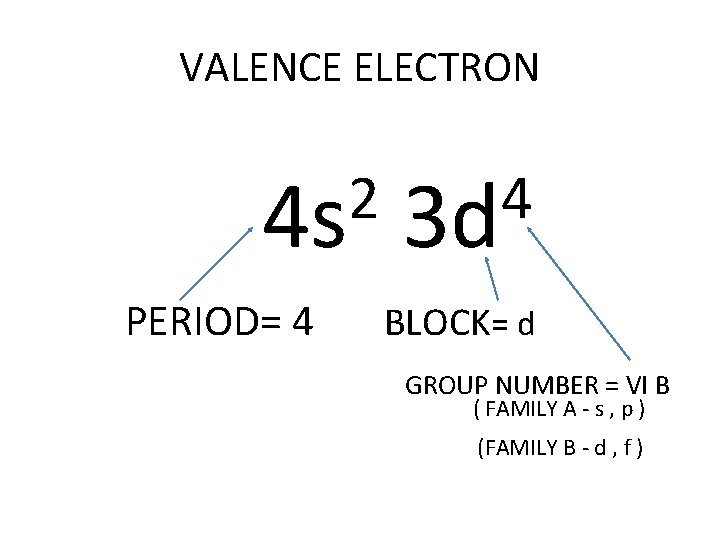
VALENCE ELECTRON 2 4 s PERIOD= 4 4 3 d BLOCK= d GROUP NUMBER = VI B ( FAMILY A - s , p ) (FAMILY B - d , f )

FAMILY B = all elements belonging to the d and f orbital 3 = IIIB 4 = IVB 5 = VB 6 = VIB 7 = VII B 8, 9, 10 = VIIIB 11 = IB 12 = 2 B
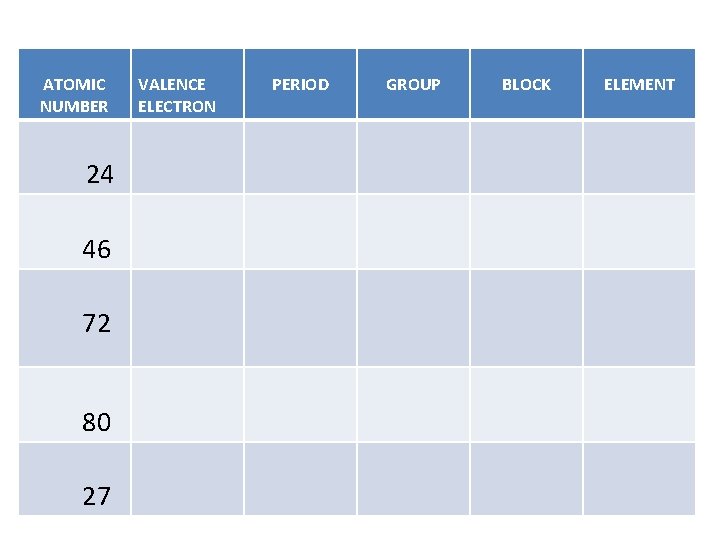
ATOMIC NUMBER 24 46 72 80 27 VALENCE ELECTRON PERIOD GROUP BLOCK ELEMENT
- Slides: 50