GSK 3 Modulates Excess NutrientInduced Autophagy Impairment in







- Slides: 14

GSK 3β Modulates Excess Nutrient-Induced Autophagy Impairment in Human Aortic Endothelial Cells via FOXO 1 Karen A. Weikel, Neil B. Ruderman, Yasuo Ido

Abstract Dysregulated autophagy, the process by which damaged proteins and organelles are turned over, and decreased AMP-activated protein kinase (AMPK) activity are each associated with type 2 diabetes and atherogenesis. In a human aortic endothelial cell (HAEC) model of poorly-controlled type 2 diabetes, we observed that the same concentrations of excess nutrients that increased inflammation and apoptosis also decreased AMPK activity and autophagy. Our current objective was to determine whether glycogen synthase kinase 3β (GSK 3β), an enzyme whose hyperactivity has been associated with diabetes, atherogenesis and inhibition of AMPK activity, attenuates autophagy in nutrient-laden HAECs. AMPK activity was measured by protein levels of p-AMPKThr 172 and the SAMS peptide assay. GSK 3β activity was measured by protein levels of p-GSK 3βSer 9, and its downstream target p-GSSer 641. GSK 3β activity was increased by treating cells with Akt inhibitor VIII (Akti) and GSK 3β activity was suppressed by infecting HAECs with lentivirus knocking down GSK 3β (sh. GSK 3β), expressing the kinase-dead (K 85 A) mutant, or incubating cells with the GSK 3β inhibitor CHIR 99021. Changes in Lysotracker staining reflected changes in lysosome acidity. Autophagosome formation was evaluated by measuring bafilomycin -induced changes in protein levels of LC 3 -II, by both Western blot and immunofluorescence. P-FOXO 1 Thr 24 and m. RNA levels of PI 3 KC 3 and SOD 2, FOXO 1 target genes, were used to evaluate FOXO 1 signaling. A combination of 25 m. M glucose and 0. 4 m. M palmitate (excess nutrient conditions) inhibited basal autophagy and AMPK activity and also increased GSK 3β activity. Treatment of HAECs with Akti decreased Lysotracker staining under both control and excess nutrient conditions. Suppression of GSK 3β activity attenuated excess nutrient-induced losses in Lysotracker staining. Under both control and excess nutrient conditions, suppression of GSK 3β activity increased LC 3 -II protein levels and knockdown of GSK 3β increased FOXO 1 signaling. These data suggest that suppression of GSK 3β activity may help restore impaired autophagy in our HAEC model of poorly-controlled type 2 diabetes by increasing lysosome acidity and autophagosome formation. The mechanism by which GSK 3β affects each of these aspects of autophagy remains to be clarified, but may involve modulation of FOXO 1 signaling.

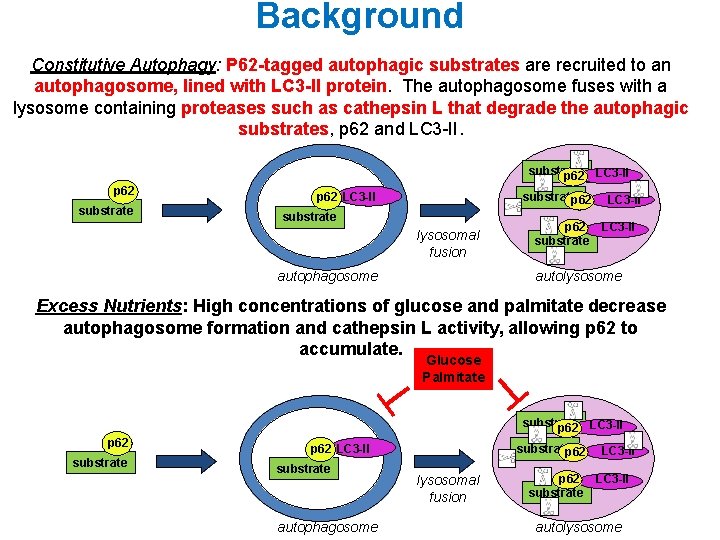
Background Constitutive Autophagy: P 62 -tagged autophagic substrates are recruited to an autophagosome, lined with LC 3 -II protein. The autophagosome fuses with a lysosome containing proteases such as cathepsin L that degrade the autophagic substrates, p 62 and LC 3 -II. substrate p 62 LC 3 -II substratep 62 substrate lysosomal fusion autophagosome p 62 substrate LC 3 -II autolysosome Excess Nutrients: High concentrations of glucose and palmitate decrease autophagosome formation and cathepsin L activity, allowing p 62 to accumulate. Glucose Palmitate substrate p 62 LC 3 -II substrate autophagosome substratep 62 lysosomal fusion p 62 substrate LC 3 -II autolysosome

Our Hypothesis Glucose Palmitate GSK 3β substrate p 62 LC 3 -II substratep 62 substrate lysosomal fusion autophagosome p 62 substrate LC 3 -II autolysosome Glucose and palmitate decrease autophagosome formation and lysosome function by increasing GSK 3β activity.

Methods • Primary human aortic endothelial cells (HAECs) were cultured in EGM-2 media. Those incubated in excess nutrient conditions were grown for at least 2 passages in EGM-2 media containing 25 m. M glucose. Confluent HAECs were then incubated in DMEM containing 5% FBS for treatment with BSA or BSA-palmitate conjugates for 6 hours. • Lysates were analyzed via western blot for expression levels of p 62, p-AMPKThr 172, t-AMPK, p-GSK 3βSer 9 , t. GSK 3β, p-GSSer 641, LC 3 -II, p-Akt. Ser 473, t-Akt, p-m. TORC 1 Ser 2448, t-m. TORC 1, p-FOXO 1 Thr 24, actin and GAPDH. • AMPK activity was measured by protein levels of p-AMPKThr 172 and the SAMS peptide assay. • Cathepsin L activity was measured using the Magic Red Cathepsin L assay kit, according to the manufacturer’s instructions. • Lysosome acidity was evaluated by treating HAECs with Lysotracker Red DND-99 and visualizing them on a fluorescent microscope. • To visualize autophagosomes, HAECs were infected with GFP-LC 3 expressing lentivirus (Addgene #22148) at 30% confluence and LC 3 -II puncta were analyzed by fluorescence microscopy. • To knockdown GSK 3β, HAECs were infected with recombinant lentivirus plasmid expressing small-hairpin RNA targeting GSK 3β (sh. GSK 3β) at 30% confluence. GSK 3β activity was also suppressed by incubating cells with the GSK 3β inhibitor, CHIR 99021. The recombinant lentivirus plasmid expressing the kinase dead GSK 3β mutant (K 85 A) was created by subcloning human GSK 3β (BC 000251) into a p. ENTR 1 A vector and site-specific mutation was introduced by PCR. GSK 3β activity was increased by incubating cells with Akt inhibitor VIII (Akti). • m. RNA levels of PIK 3 C 3 and SOD 2 were quantified by RT-PCR using SYBR green and quantified using the 2(Delta C(T)) method, relative to actin.

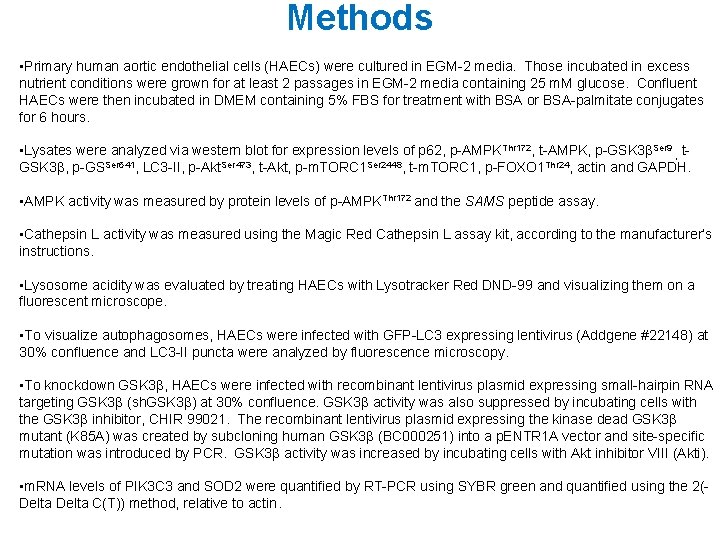
Results A * B * GFP-LC 3 5 m. M glucose (m. M) 5 palmitate (m. M) 0 rapamycin (µM) 0 25 0. 4 10 10 n. M bafilomycin 25 m. M glucose 0. 4 m. M palmitate 10 n. M bafilomycin 10 µM rapamycin * * p 62 actin C * glucose (m. M) palmitate (m. M) 5 0 25 0. 4 glucose (m. M) 5 palmitate (m. M) 0 rapamycin (µM) 0 25 0. 4 10 Figure 1. Excess nutrients decrease autophagy.

A C * glucose (m. M) palmitate (m. M) 5 0 * * 25 0. 4 glucose (m. M) palmitate (m. M) p-AMPK α 1/α 2 Thr 172 t-AMPK α 1 5 0 25 0. 4 p-GSSer 641 actin p-GSK 3βSer 9 B t-GSK 3β GAPDH * glucose (m. M) palmitate (m. M) 5 0 25 0. 4 Figure 2. Excess nutrients decrease AMPK activity and increase GSK 3β activity.

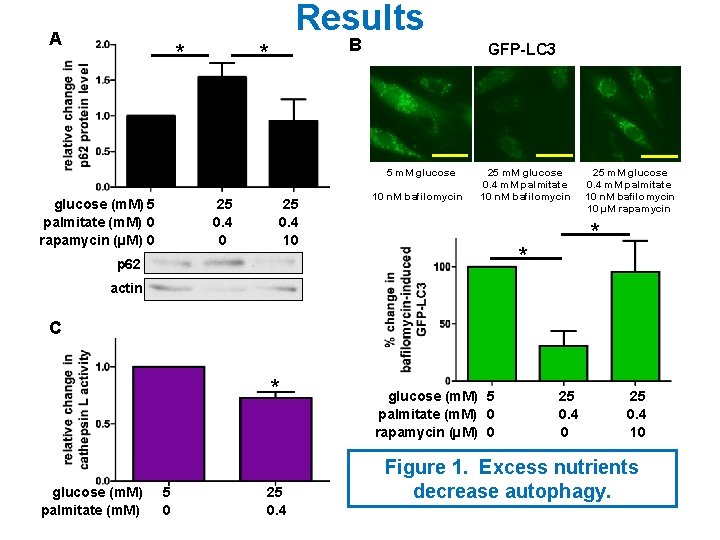
A Control Conditions glucose (m. M) palmitate (m. M) Akti (µM) 5 0 0 5 0 40 - Akti + Akti p-GSK 3βSer 9 * GAPDH glucose (m. M) 5 palmitate (m. M) 0 Akti (µM) 0 B 5 0 40 Excess Nutrient Conditions glucose (m. M) 25 palmitate (m. M) 0. 4 Akti (µM) 0 25 0. 4 40 - Akti + Akti p-GSK 3βSer 9 GAPDH Figure 3. Akti decreases Lysotracker staining. * glucose (m. M) 25 palmitate (m. M) 0. 4 Akti (µM) 0 25 0. 4 40

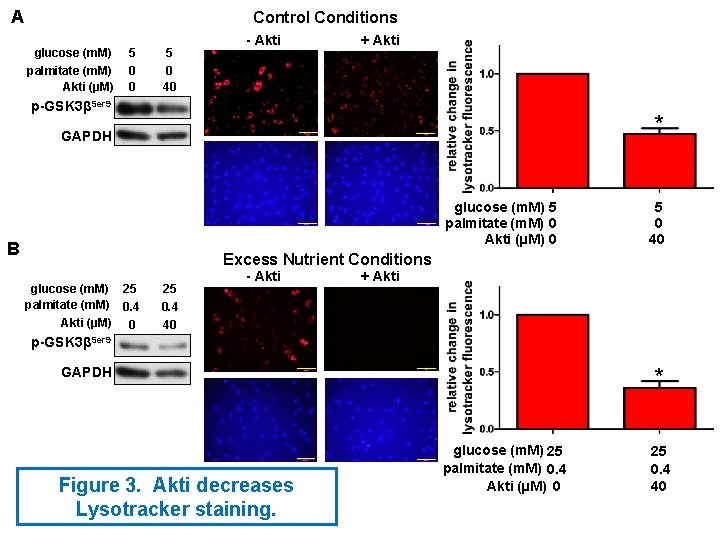
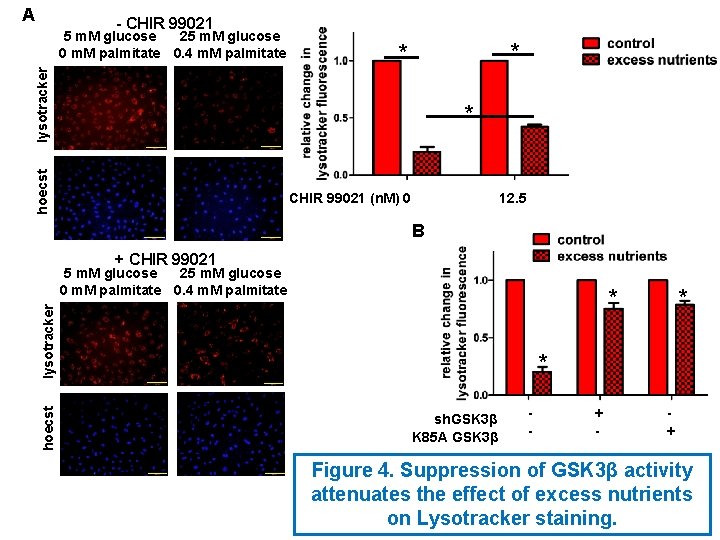
A - CHIR 99021 * * lysotracker 5 m. M glucose 25 m. M glucose 0 m. M palmitate 0. 4 m. M palmitate hoecst * CHIR 99021 (n. M) 0 12. 5 B + CHIR 99021 5 m. M glucose 25 m. M glucose 0 m. M palmitate 0. 4 m. M palmitate hoecst lysotracker * * * sh. GSK 3β K 85 A GSK 3β - + Figure 4. Suppression of GSK 3β activity attenuates the effect of excess nutrients on Lysotracker staining.

A 5 m. M glucose 25 m. M glucose 0 m. M palmitate 0. 4 m. M palmitate 10 n. M bafilomycin glucose (m. M) 5 palmitate (m. M) 0 bafilomycin (n. M) 10 B 25 0. 4 10 * * sh. GSK 3β * shc * sh. GSK 3β shc glucose (m. M) palmitate (m. M) bafilomycin (n. M) + 5 0 0 + + - 5 5 0 0 0 10 + 5 0 10 + 25 0. 4 0 - + + 25 25 0. 4 10 0 0 10 LC 3 -II t-GSK 3β glucose (m. M) 5 palmitate (m. M) 0 25 0. 4 GAPDH Figure 5. GSK 3β knockdown increases LC 3 -II protein levels under both control and nutrient excess conditions.

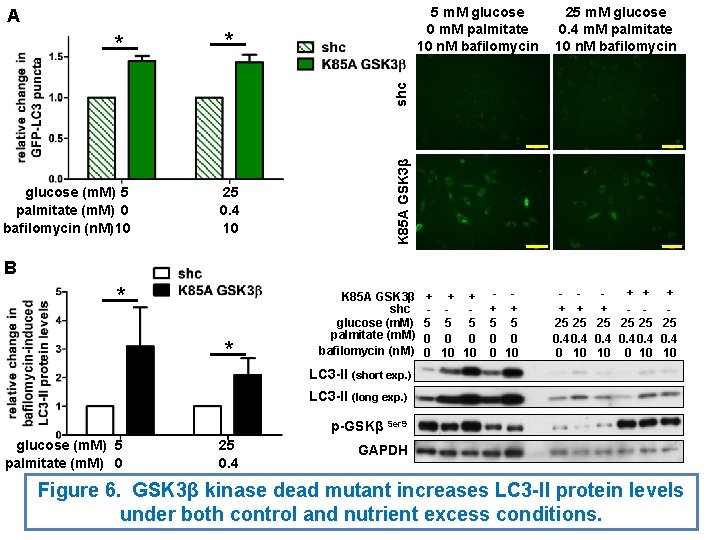
5 m. M glucose 0 m. M palmitate 10 n. M bafilomycin A * glucose (m. M) 5 palmitate (m. M) 0 bafilomycin (n. M) 10 25 0. 4 10 K 85 A GSK 3β shc * 25 m. M glucose 0. 4 m. M palmitate 10 n. M bafilomycin B * * K 85 A GSK 3β shc glucose (m. M) palmitate (m. M) bafilomycin (n. M) + 5 0 0 + + 5 5 0 0 10 10 - + + 5 5 0 0 0 10 - + + + - 25 25 25 0. 4 0 10 10 LC 3 -II (short exp. ) LC 3 -II (long exp. ) p-GSKβ Ser 9 glucose (m. M) 5 palmitate (m. M) 0 25 0. 4 GAPDH Figure 6. GSK 3β kinase dead mutant increases LC 3 -II protein levels under both control and nutrient excess conditions.

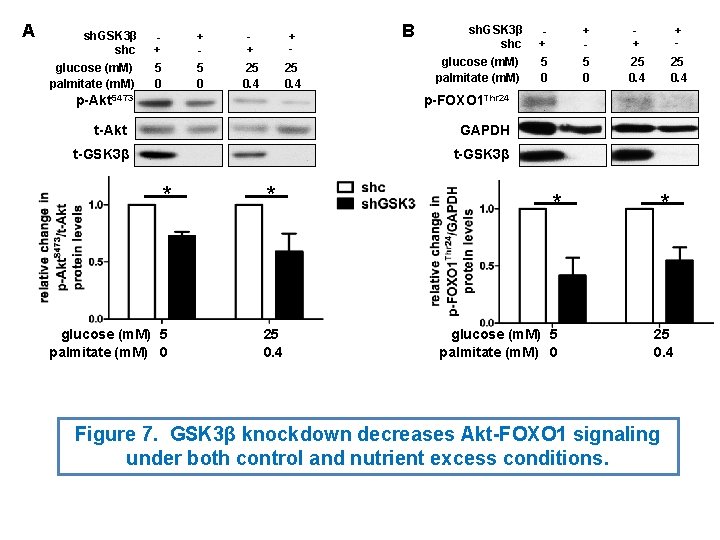
A sh. GSK 3β shc glucose (m. M) palmitate (m. M) + 5 0 + 25 0. 4 B sh. GSK 3β shc glucose (m. M) palmitate (m. M) + 5 0 + 25 0. 4 p-FOXO 1 Thr 24 p-Akt. S 473 t-Akt GAPDH t-GSK 3β * glucose (m. M) 5 palmitate (m. M) 0 * 25 0. 4 Figure 7. GSK 3β knockdown decreases Akt-FOXO 1 signaling under both control and nutrient excess conditions.

A B * glucose (m. M) 5 palmitate (m. M) 0 * 25 0. 4 Figure 8. GSK 3β knockdown increases m. RNA levels of FOXO 1 targeted genes.

Conclusions • Exposure of HAECs to excess nutrients decreases autophagy, decreases AMPK activity and increases GSK 3β activity. • Excess nutrients decrease lysosome acidification in a GSK 3β-dependent manner. • Knockdown of GSK 3β increases autophagosome formation under both control and excess nutrient conditions. • • Knockdown of GSK 3β increases FOXO 1 signaling. Suppression of GSK 3β activity may improve aortic endothelial cell homeostasis in type 2 diabetes by attenuating nutrient-induced damage of lysosomes and autophagosomes. This mechanism may involve modulation of both AMPK and FOXO 1 activities. GSK 3β inhibitor Excess nutrientinduced GSK 3β activity Akt activity AMPK activity FOXO 1 activity autophagosome formation
 Autophagy
Autophagy Smooth endoplasmic reticulum function
Smooth endoplasmic reticulum function Albendazole gsk
Albendazole gsk Gsk sanofi
Gsk sanofi Gsk mission
Gsk mission Gsk respiratorio
Gsk respiratorio Torno gsk
Torno gsk Setproxy.gsk.com
Setproxy.gsk.com Gsk glaxosmithkline
Gsk glaxosmithkline Gsk.com careers
Gsk.com careers Accommodations for speech and language impairment
Accommodations for speech and language impairment Impairment vs disability
Impairment vs disability Impairment related work expenses
Impairment related work expenses Workplace impairment
Workplace impairment Northern speech services
Northern speech services