GroupsFamilies The vertical columns of the periodic table

Groups/Families • The vertical columns of the periodic table correspond to the groups or families of chemicals • Really useful information when combined with other periodic trends

Alkali Metals • 1 st column: Li, Na, K, Rb, Cs, Fr • Have one valance electron • Not found alone in nature – React violently with water to produce H 2 gas and a strong base (OH-) • Strong bases have a high p. H – Na and K are crucial for nerve impulse

Alkaline-Earth Metals • 2 nd column: Be, Mg, Ca, Sr, Ba, Ra • Two valence electrons • Not found alone in nature – React strongly with water to produce H 2 gas and a strong base • Base is not as strong – lower p. H
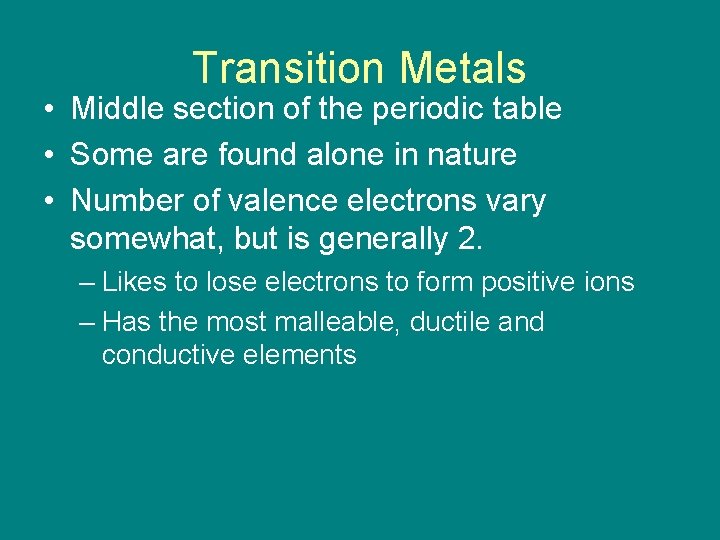
Transition Metals • Middle section of the periodic table • Some are found alone in nature • Number of valence electrons vary somewhat, but is generally 2. – Likes to lose electrons to form positive ions – Has the most malleable, ductile and conductive elements

Lanthanide and Actinide Series • Inner Transition Metals – Named because they begin with La and Ac – Fit between the Alkaline Earth and Transition Metals, but are placed below to save space – All Actinides are radioactive – Cerium, a Lanthanide, is quite common.

Boron Family • B, Al, Ga, In, Tl # 113 • 3 valence electrons • Generally not found alone in nature. – Form really stable compounds with oxygen – Aluminum is the most common metal on earth

Carbon Family • C, Si Ge, Sn, Pb • Four valence electrons • Can be found alone in nature – Carbon is found in more compounds than any other element – Organic chemistry – Carbon and silicon have different allotropes

Nitrogen Family • N, P, As, Sb, Bi • 5 valence electrons • Can be found alone in nature – Nitrogen takes up 78% of the Earth’s atmosphere – Nitrogen is found in all living things, but not usually in the most stable form of N 2.

Oxygen Group • • • O, S, Se, Te, Po 6 valence electrons Can be found alone in nature Oxygen is required for aerobic respiration Some bacteria use S for anaerobic respiration.
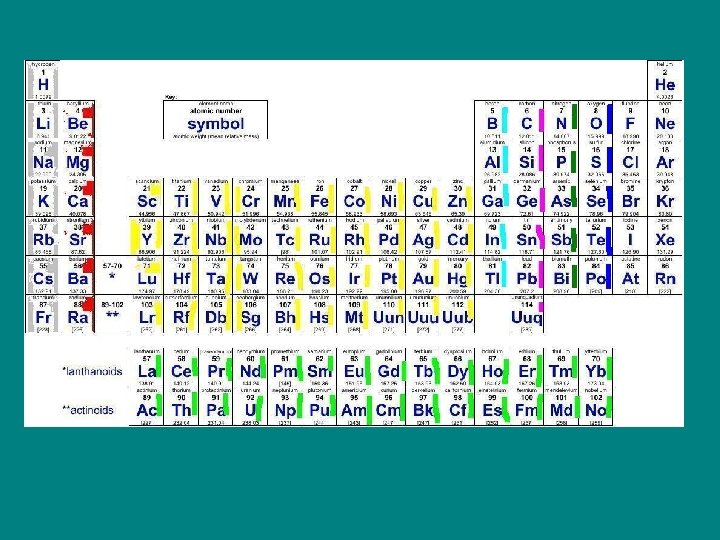

Halogens • F, Cl , Br, I, At • 7 valence electrons • Not found alone in nature – Highly reactive with alkalis form salts. • Halo- means salt – H + halogen can make a strong acid – Used as disinfectants and strengthens teeth (I, Br, Cl and Fl)
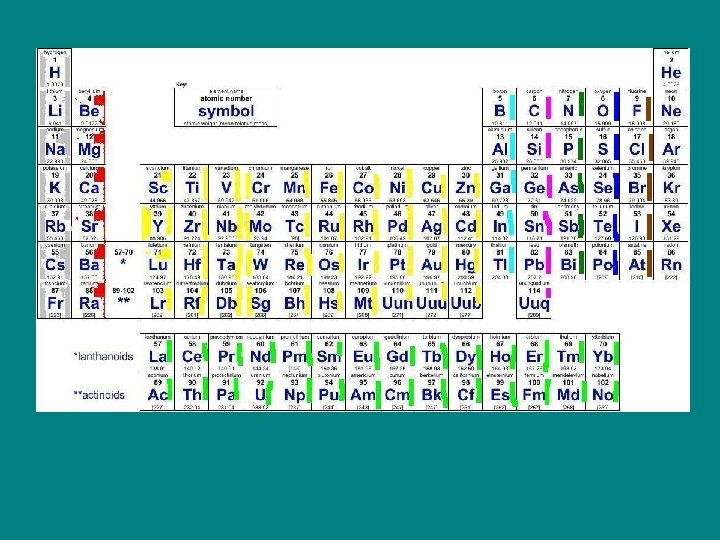

Noble Gasses • He, Ne, Ar, Kr, Xe, Rn • Eight valence electrons • Always found alone in nature – All occur naturally. – Radon is known to cause cancer and can reach high levels in basements of houses. – He is used in balloons. All of the gasses can be used to make “neon” lights
- Slides: 22