GROUP VII The Halogens A guide for GCSE



















- Slides: 58

GROUP VII The Halogens A guide for GCSE students KNOCKHARDY PUBLISHING 2010 SPECIFICATIONS

GROUP VII INTRODUCTION This Powerpoint show is one of several produced to help students understand selected GCSE Chemistry topics. It is based on the requirements of the AQA specification but is suitable for other examination boards. Individual students may use the material at home for revision purposes and it can also prove useful for classroom teaching with an interactive white board. Additional Powerpoints, and the full range of AS and A 2 Chemistry topics, are available from the KNOCKHARDY WEBSITE at. . . www. knockhardy. org. uk All diagrams and animations in this Powerpoint are original and created by Jonathan Hopton. Permission must be obtained for their use in any commercial work.

GROUP VII CONTENTS • Introduction • Group trends • Group similarities • Reaction with metals • Displacement reactions • Summary • Quick quiz

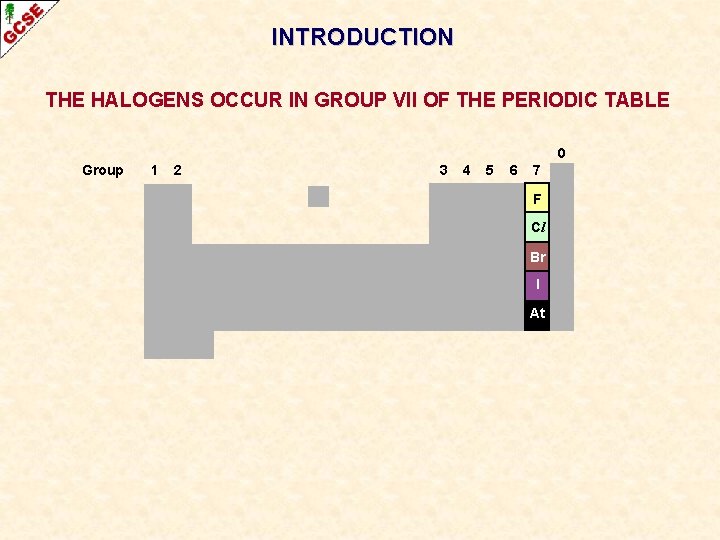
INTRODUCTION THE HALOGENS OCCUR IN GROUP VII OF THE PERIODIC TABLE 0 Group 1 2 3 4 5 6 7 F Cl Br I At

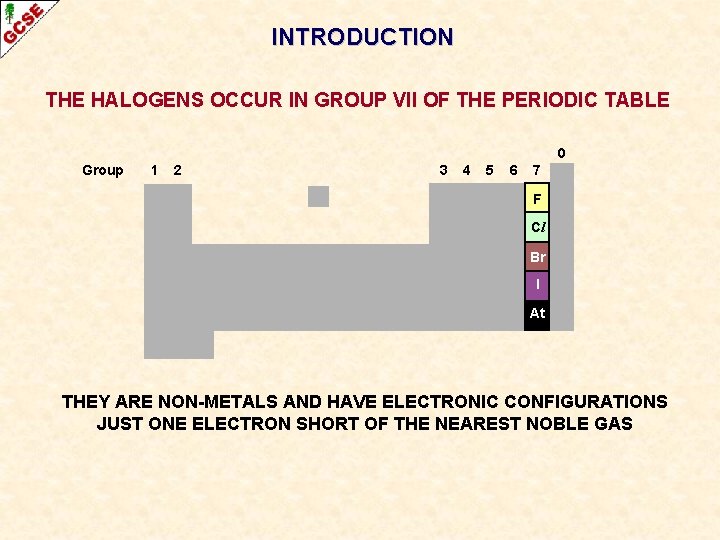
INTRODUCTION THE HALOGENS OCCUR IN GROUP VII OF THE PERIODIC TABLE 0 Group 1 2 3 4 5 6 7 F Cl Br I At THEY ARE NON-METALS AND HAVE ELECTRONIC CONFIGURATIONS JUST ONE ELECTRON SHORT OF THE NEAREST NOBLE GAS

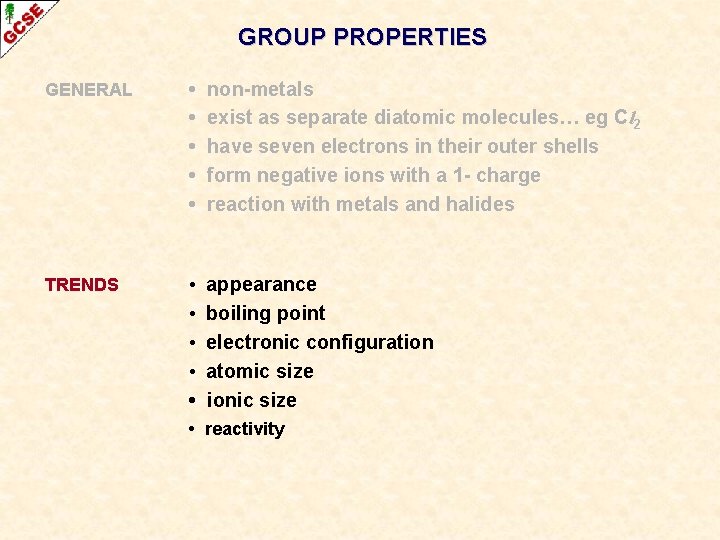
GROUP PROPERTIES GENERAL • • • non-metals exist as separate diatomic molecules… eg Cl 2 have seven electrons in their outer shells form negative ions with a 1 - charge reaction with metals and halides

GROUP PROPERTIES GENERAL • • • non-metals exist as separate diatomic molecules… eg Cl 2 have seven electrons in their outer shells form negative ions with a 1 - charge reaction with metals and halides TRENDS • • • appearance boiling point electronic configuration atomic size ionic size • reactivity

GROUP TRENDS

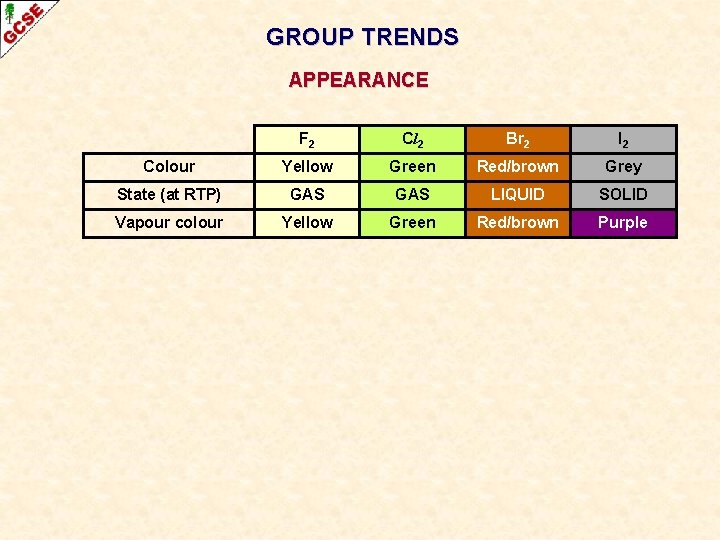
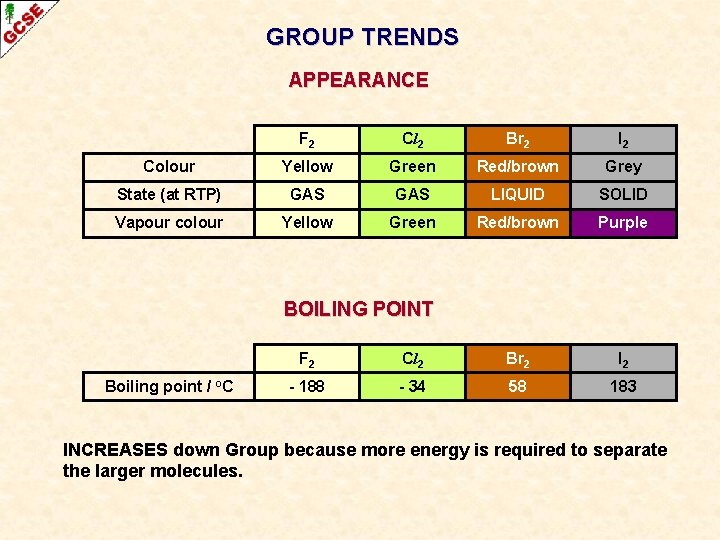
GROUP TRENDS APPEARANCE F 2 Cl 2 Br 2 I 2 Colour Yellow Green Red/brown Grey State (at RTP) GAS LIQUID SOLID Vapour colour Yellow Green Red/brown Purple

GROUP TRENDS APPEARANCE F 2 Cl 2 Br 2 I 2 Colour Yellow Green Red/brown Grey State (at RTP) GAS LIQUID SOLID Vapour colour Yellow Green Red/brown Purple BOILING POINT Boiling point / °C F 2 Cl 2 Br 2 I 2 - 188 - 34 58 183 INCREASES down Group because more energy is required to separate the larger molecules.

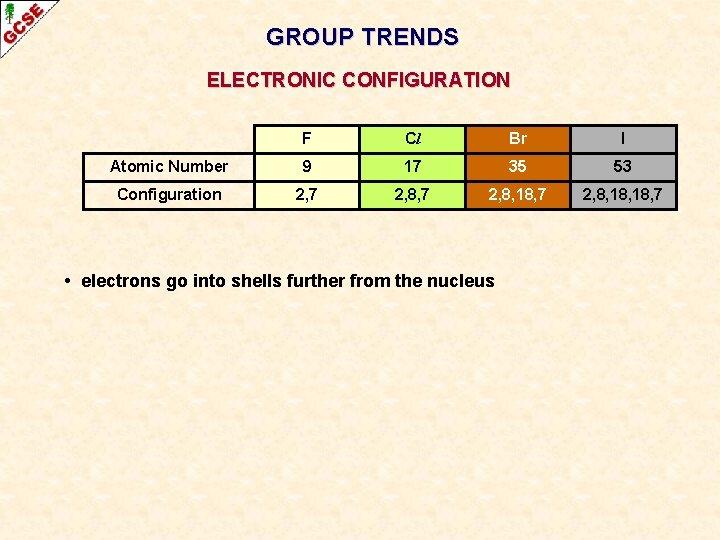
GROUP TRENDS ELECTRONIC CONFIGURATION F Cl Br I Atomic Number 9 17 35 53 Configuration 2, 7 2, 8, 18, 7 • electrons go into shells further from the nucleus

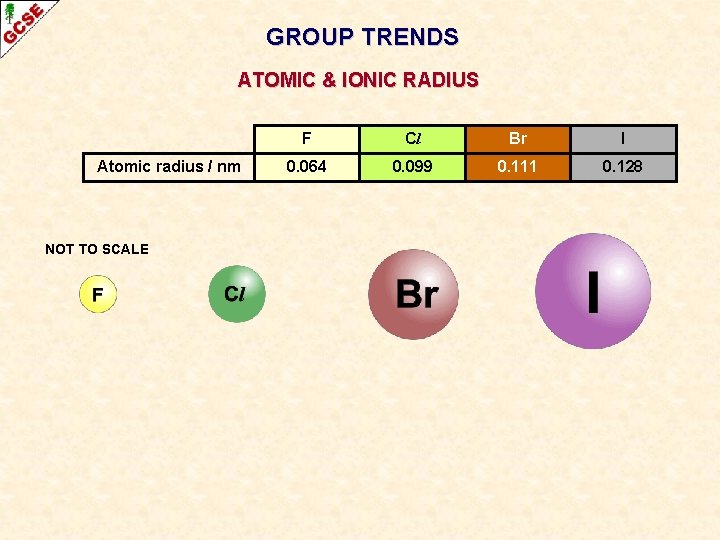
GROUP TRENDS ATOMIC & IONIC RADIUS Atomic radius / nm NOT TO SCALE F Cl Br I 0. 064 0. 099 0. 111 0. 128

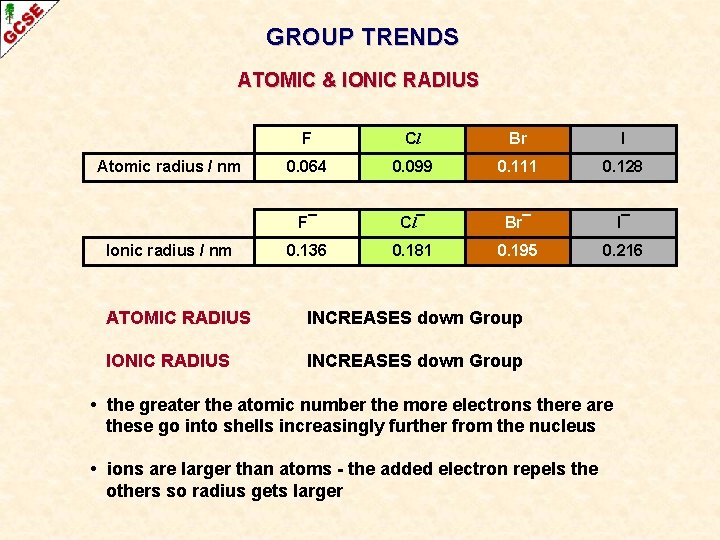
GROUP TRENDS ATOMIC & IONIC RADIUS Atomic radius / nm Ionic radius / nm F Cl Br I 0. 064 0. 099 0. 111 0. 128 F¯ Cl¯ Br¯ I¯ 0. 136 0. 181 0. 195 0. 216 ATOMIC RADIUS INCREASES down Group IONIC RADIUS INCREASES down Group • the greater the atomic number the more electrons there are these go into shells increasingly further from the nucleus • ions are larger than atoms - the added electron repels the others so radius gets larger

GROUP SIMILARITIES

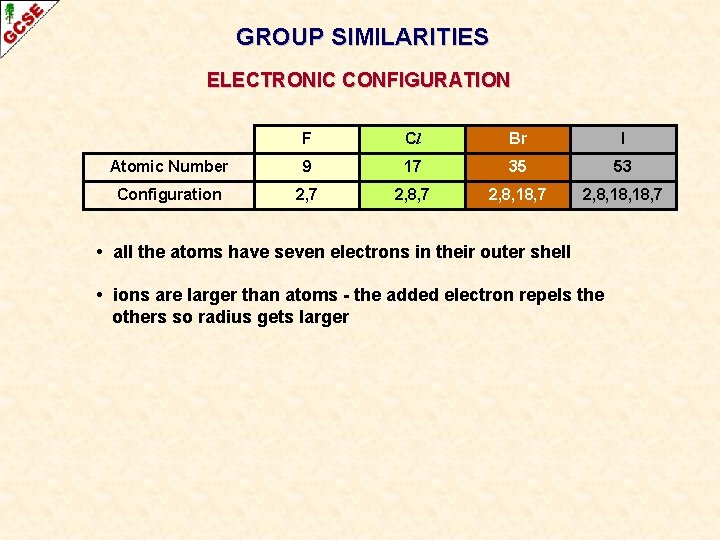
GROUP SIMILARITIES ELECTRONIC CONFIGURATION F Cl Br I Atomic Number 9 17 35 53 Configuration 2, 7 2, 8, 18, 7 • all the atoms have seven electrons in their outer shell • ions are larger than atoms - the added electron repels the others so radius gets larger

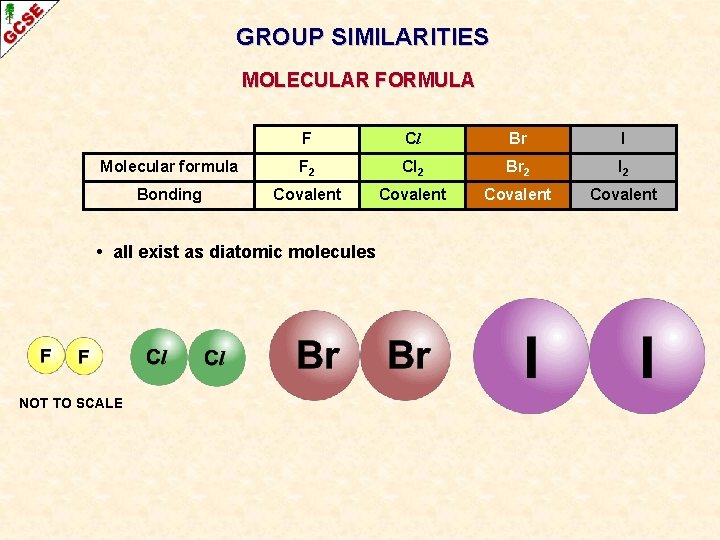
GROUP SIMILARITIES MOLECULAR FORMULA F Cl Br I Molecular formula F 2 Cl 2 Br 2 I 2 Bonding Covalent • all exist as diatomic molecules NOT TO SCALE

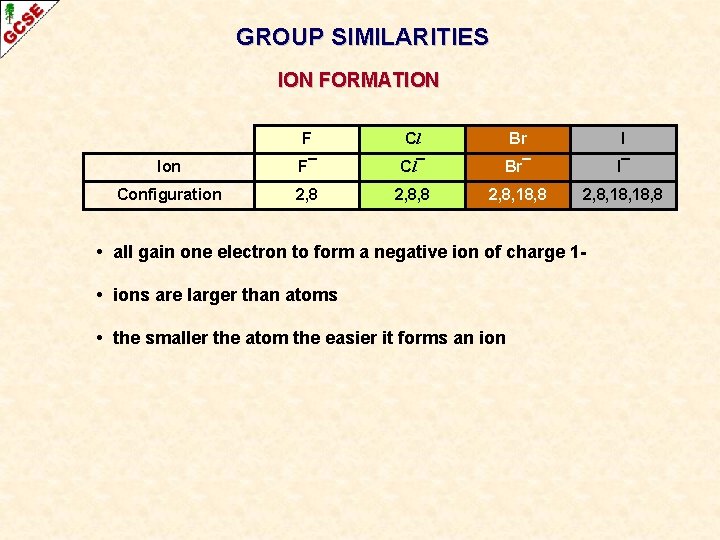
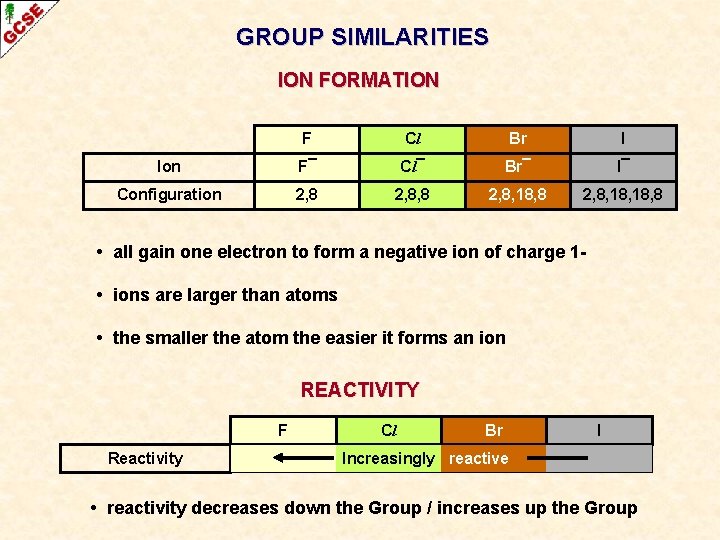
GROUP SIMILARITIES ION FORMATION F Cl Br I Ion F¯ Cl¯ Br¯ I¯ Configuration 2, 8, 8 2, 8, 18, 18, 8 • all gain one electron to form a negative ion of charge 1 • ions are larger than atoms • the smaller the atom the easier it forms an ion

GROUP SIMILARITIES ION FORMATION F Cl Br I Ion F¯ Cl¯ Br¯ I¯ Configuration 2, 8, 8 2, 8, 18, 18, 8 • all gain one electron to form a negative ion of charge 1 • ions are larger than atoms • the smaller the atom the easier it forms an ion REACTIVITY F Reactivity Cl Br I Increasingly reactive • reactivity decreases down the Group / increases up the Group
REACTIONS OF HALOGENS 1. WITH METALS 2. WITH HALIDES

REACTION OF HALOGENS WITH METALS

REACTION OF HALOGENS WITH METALS HALOGENS REACT WITH METALS TO PRODUCE METAL HALIDES.

REACTION OF HALOGENS WITH METALS HALOGENS REACT WITH METALS TO PRODUCE METAL HALIDES. THE EASE OF REACTION DECREASES DOWN THE GROUP F > Cl > Br > I

REACTION OF HALOGENS WITH METALS HALOGENS REACT WITH METALS TO PRODUCE METAL HALIDES. THE EASE OF REACTION DECREASES DOWN THE GROUP F > Cl > Br > I THIS IS BECAUSE ‘THE LARGER THE HALOGEN ATOM, THE LESS EASILY IT ATTRACTS THE ELECTRON IT NEEDS TO FILL ITS OUTER SHELL’

REACTION OF HALOGENS WITH METALS HALOGENS REACT WITH METALS TO PRODUCE METAL HALIDES. THE EASE OF REACTION DECREASES DOWN THE GROUP F > Cl > Br > I THIS IS BECAUSE ‘THE LARGER THE HALOGEN ATOM, THE LESS EASILY IT ATTRACTS THE ELECTRON IT NEEDS TO FILL ITS OUTER SHELL’ THE HALIDES OF GROUP I ARE… WHITE IONIC SOLIDS VERY SOLUBLE IN WATER SODIUM CHLORIDE (Na. Cl) IS A TYPICAL GROUP I HALIDE

REACTION WITH ALKALI METALS

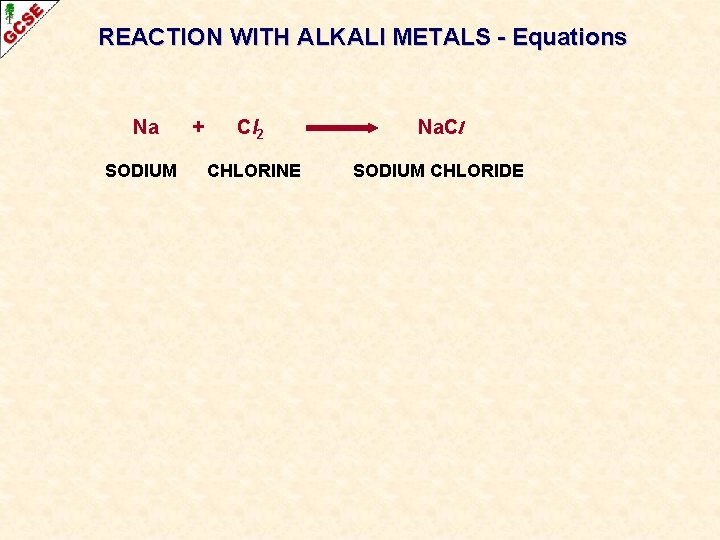
REACTION WITH ALKALI METALS - Equations SODIUM + CHLORINE SODIUM CHLORIDE

REACTION WITH ALKALI METALS - Equations Na SODIUM + Cl 2 Na. Cl CHLORINE SODIUM CHLORIDE

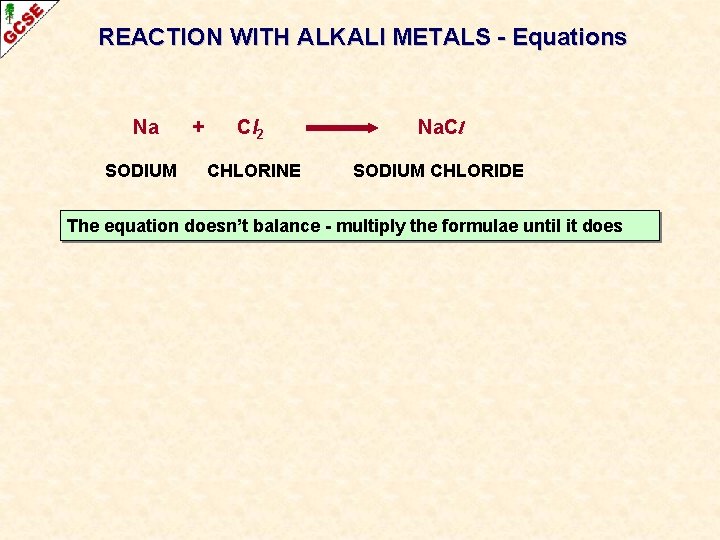
REACTION WITH ALKALI METALS - Equations Na SODIUM + Cl 2 Na. Cl CHLORINE SODIUM CHLORIDE The equation doesn’t balance - multiply the formulae until it does

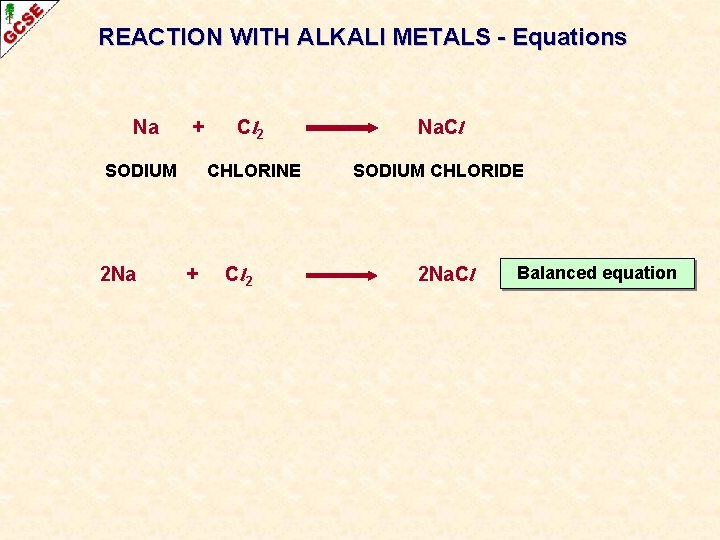
REACTION WITH ALKALI METALS - Equations Na + SODIUM 2 Na + Cl 2 Na. Cl CHLORINE SODIUM CHLORIDE Cl 2 2 Na. Cl Balanced equation

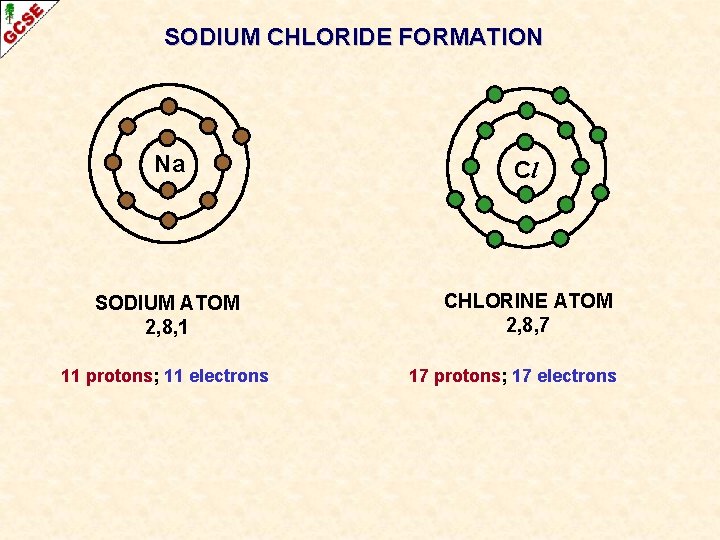
SODIUM CHLORIDE FORMATION Na Cl SODIUM ATOM 2, 8, 1 CHLORINE ATOM 2, 8, 7 11 protons; 11 electrons 17 protons; 17 electrons

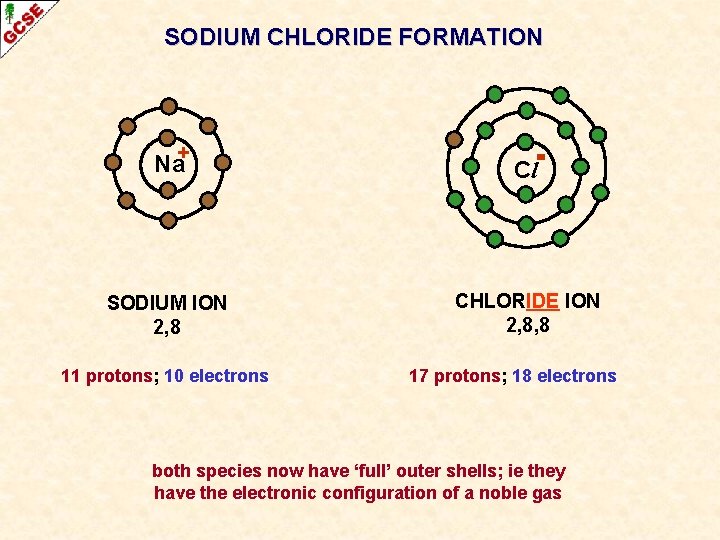
SODIUM CHLORIDE FORMATION Na+ Cl SODIUM ION 2, 8 CHLORIDE ION 2, 8, 8 11 protons; 10 electrons 17 protons; 18 electrons both species now have ‘full’ outer shells; ie they have the electronic configuration of a noble gas

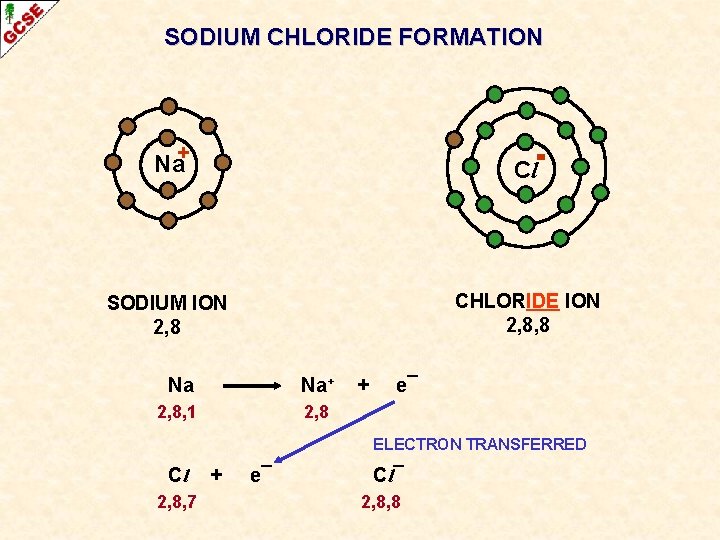
SODIUM CHLORIDE FORMATION Na+ Cl SODIUM ION 2, 8 CHLORIDE ION 2, 8, 8 Na Na+ 2, 8, 1 2, 8 + e¯ ELECTRON TRANSFERRED Cl 2, 8, 7 + e¯ Cl¯ 2, 8, 8

DISPLACEMENT REACTIONS OF HALOGENS

DISPLACEMENT REACTIONS OF HALOGENS GET LESS REACTIVE AS THE GROUP IS DESCENDED

DISPLACEMENT REACTIONS OF HALOGENS GET LESS REACTIVE AS THE GROUP IS DESCENDED THIS DECREASE IN REACTIVITY DOWN THE GROUP CAN BE DEMONSTRATED USING DISPLACEMENT REACTIONS. . . A DISPLACEMENT REACTION IS WHERE ONE SPECIES TAKES THE PLACE OF ANOTHER IN A COMPOUND.

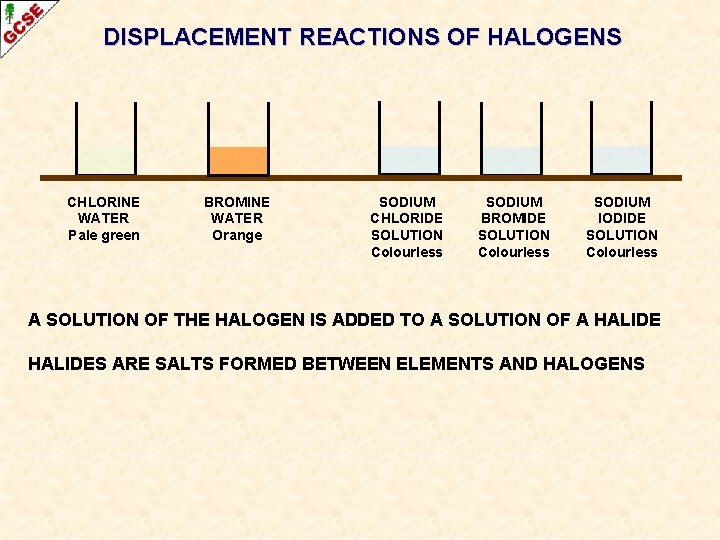
DISPLACEMENT REACTIONS OF HALOGENS CHLORINE WATER Pale green BROMINE WATER Orange SODIUM CHLORIDE SOLUTION Colourless SODIUM BROMIDE SOLUTION Colourless SODIUM IODIDE SOLUTION Colourless A SOLUTION OF THE HALOGEN IS ADDED TO A SOLUTION OF A HALIDES ARE SALTS FORMED BETWEEN ELEMENTS AND HALOGENS

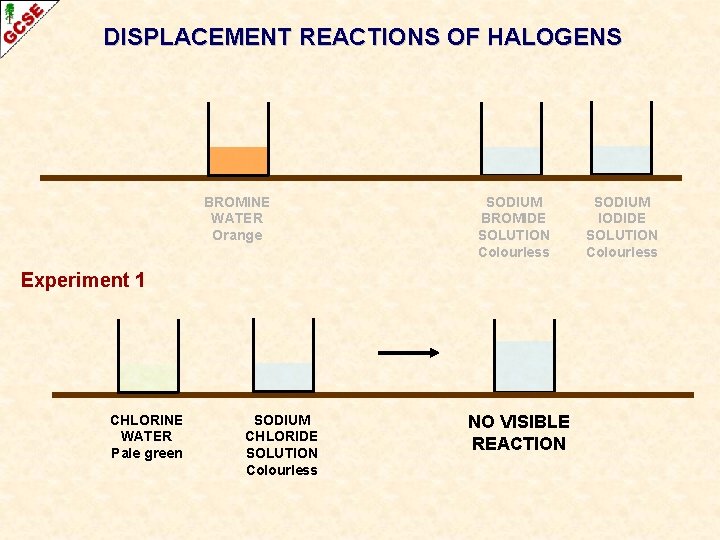
DISPLACEMENT REACTIONS OF HALOGENS BROMINE WATER Orange SODIUM BROMIDE SOLUTION Colourless Experiment 1 CHLORINE WATER Pale green SODIUM CHLORIDE SOLUTION Colourless NO VISIBLE REACTION SODIUM IODIDE SOLUTION Colourless

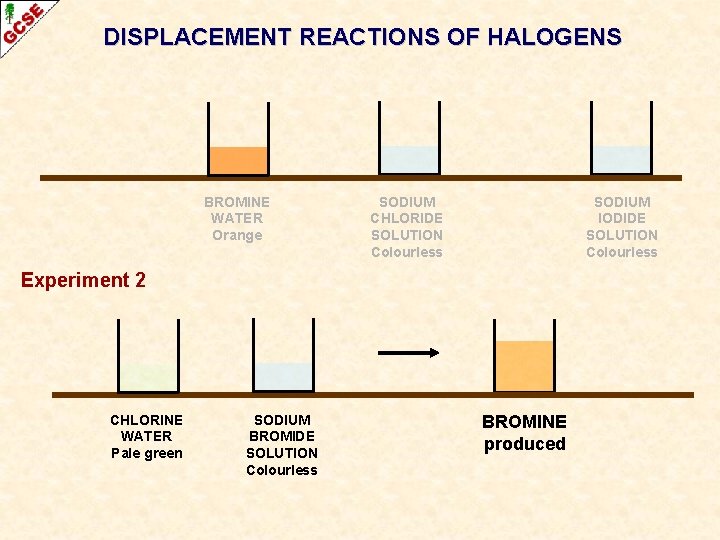
DISPLACEMENT REACTIONS OF HALOGENS BROMINE WATER Orange SODIUM CHLORIDE SOLUTION Colourless SODIUM IODIDE SOLUTION Colourless Experiment 2 CHLORINE WATER Pale green SODIUM BROMIDE SOLUTION Colourless BROMINE produced

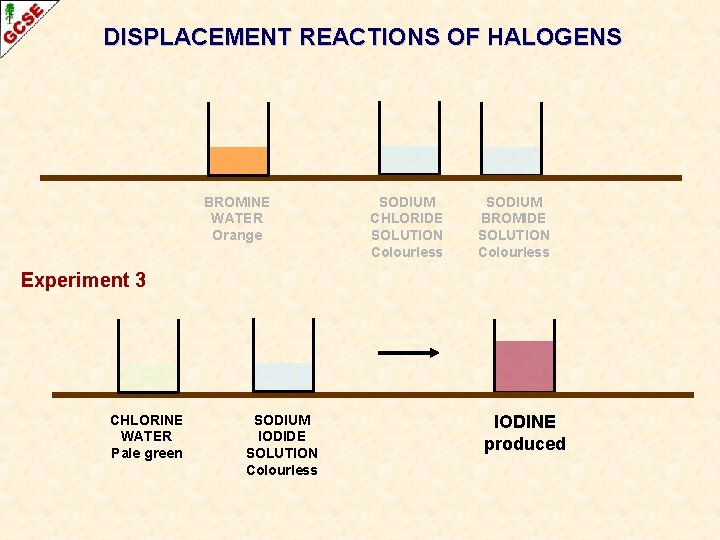
DISPLACEMENT REACTIONS OF HALOGENS BROMINE WATER Orange SODIUM CHLORIDE SOLUTION Colourless SODIUM BROMIDE SOLUTION Colourless Experiment 3 CHLORINE WATER Pale green SODIUM IODIDE SOLUTION Colourless IODINE produced

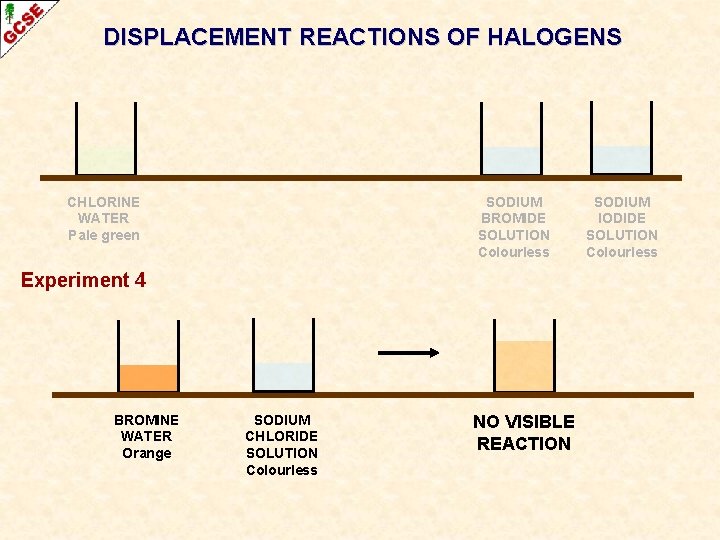
DISPLACEMENT REACTIONS OF HALOGENS CHLORINE WATER Pale green SODIUM BROMIDE SOLUTION Colourless Experiment 4 BROMINE WATER Orange SODIUM CHLORIDE SOLUTION Colourless NO VISIBLE REACTION SODIUM IODIDE SOLUTION Colourless

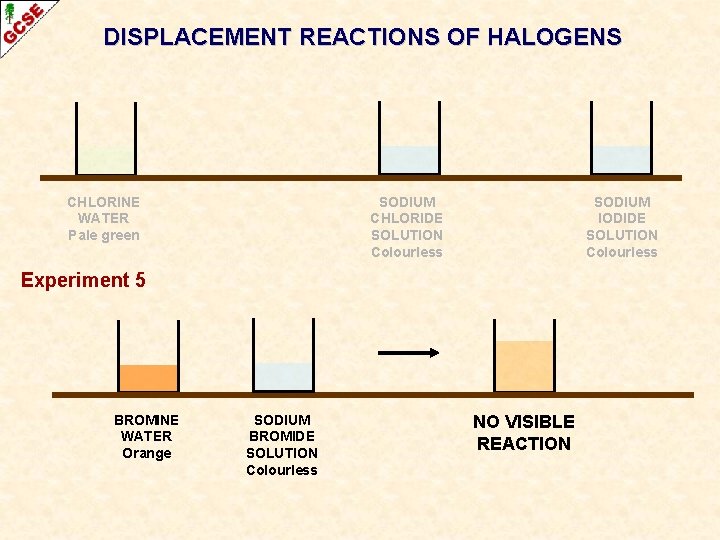
DISPLACEMENT REACTIONS OF HALOGENS CHLORINE WATER Pale green SODIUM CHLORIDE SOLUTION Colourless SODIUM IODIDE SOLUTION Colourless Experiment 5 BROMINE WATER Orange SODIUM BROMIDE SOLUTION Colourless NO VISIBLE REACTION

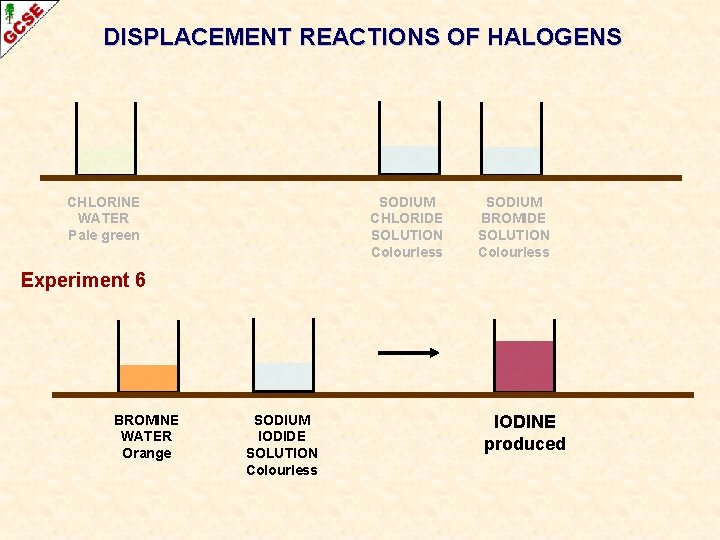
DISPLACEMENT REACTIONS OF HALOGENS CHLORINE WATER Pale green SODIUM CHLORIDE SOLUTION Colourless SODIUM BROMIDE SOLUTION Colourless Experiment 6 BROMINE WATER Orange SODIUM IODIDE SOLUTION Colourless IODINE produced

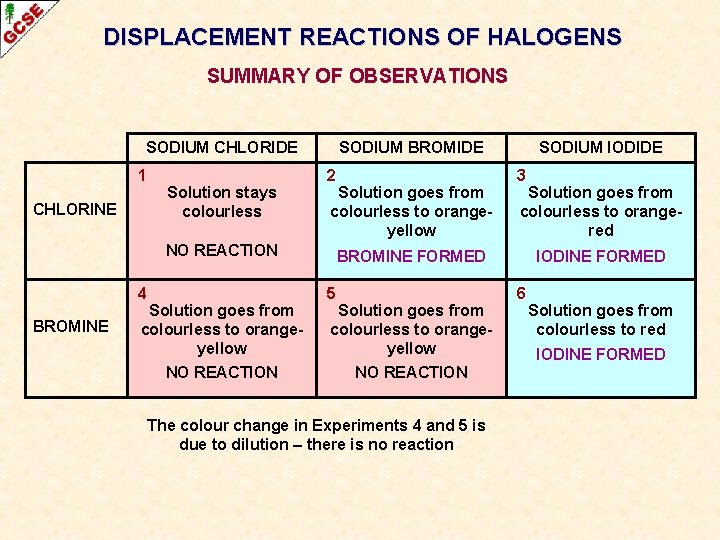
DISPLACEMENT REACTIONS OF HALOGENS SUMMARY OF OBSERVATIONS SODIUM CHLORIDE 1 CHLORINE Solution stays colourless SODIUM BROMIDE 2 Solution goes from colourless to orangeyellow NO REACTION 4 BROMINE Solution goes from colourless to orangeyellow NO REACTION SODIUM IODIDE 3 Solution goes from colourless to orangered BROMINE FORMED 5 Solution goes from colourless to orangeyellow NO REACTION The colour change in Experiments 4 and 5 is due to dilution – there is no reaction IODINE FORMED 6 Solution goes from colourless to red IODINE FORMED

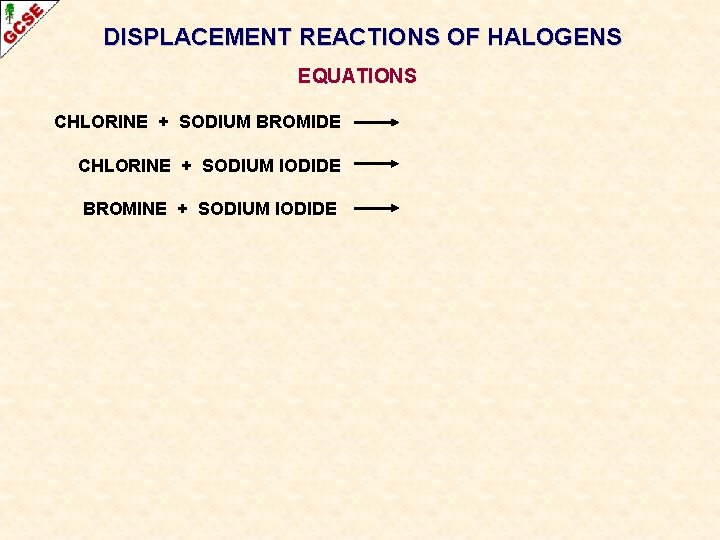
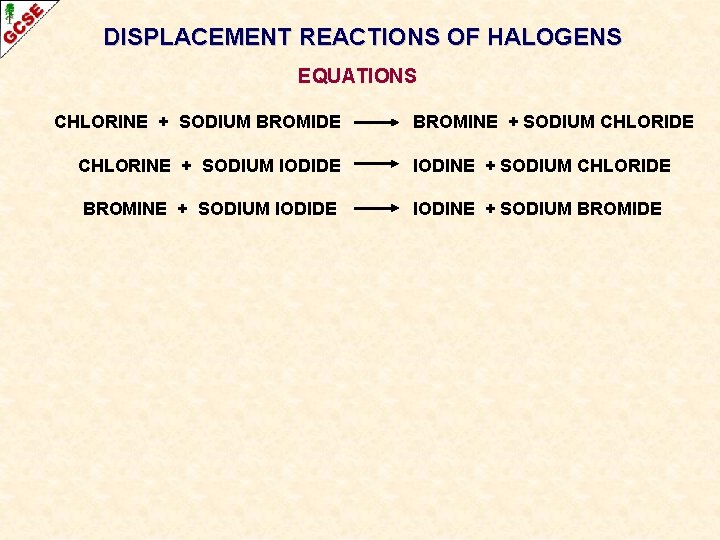
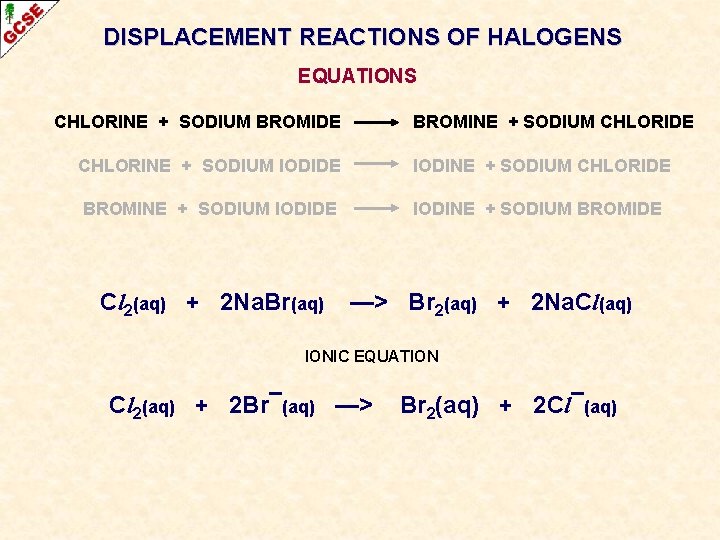
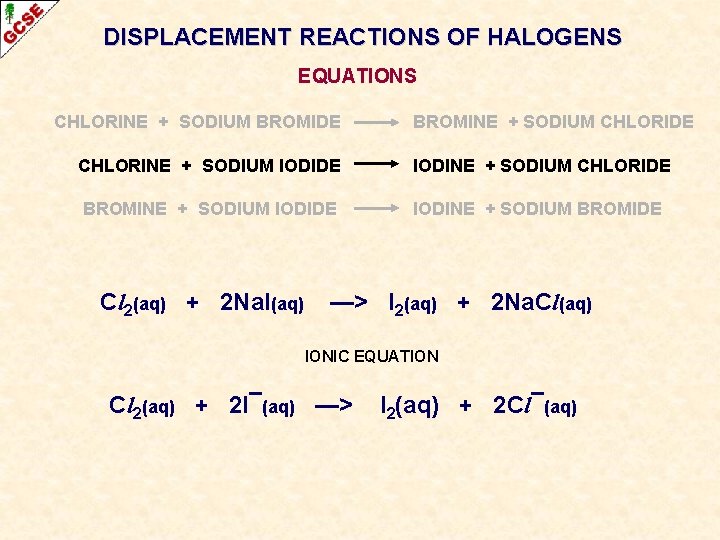
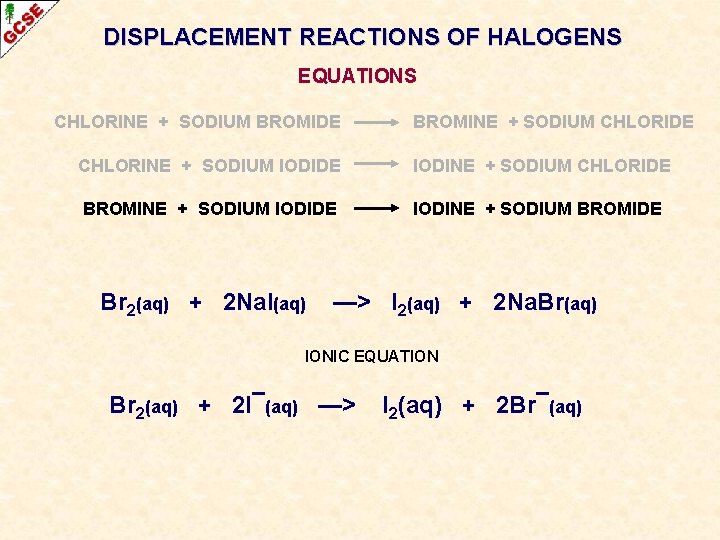
DISPLACEMENT REACTIONS OF HALOGENS EQUATIONS CHLORINE + SODIUM BROMIDE CHLORINE + SODIUM IODIDE BROMINE + SODIUM IODIDE

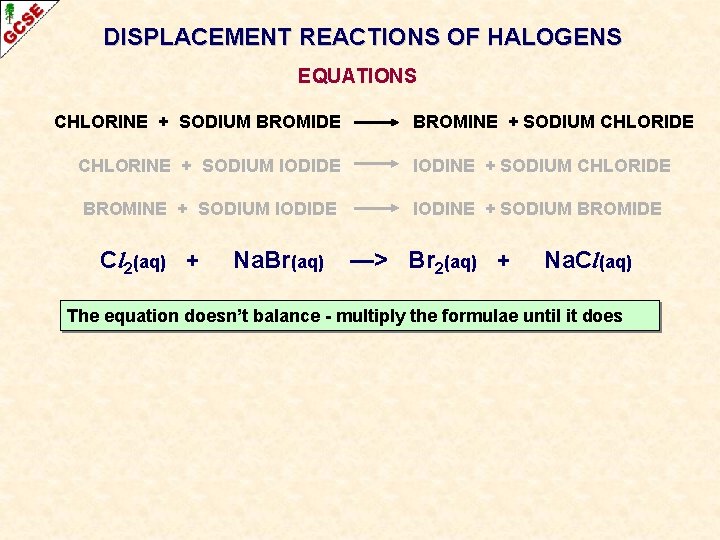
DISPLACEMENT REACTIONS OF HALOGENS EQUATIONS CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM IODIDE IODINE + SODIUM CHLORIDE BROMINE + SODIUM IODIDE IODINE + SODIUM BROMIDE

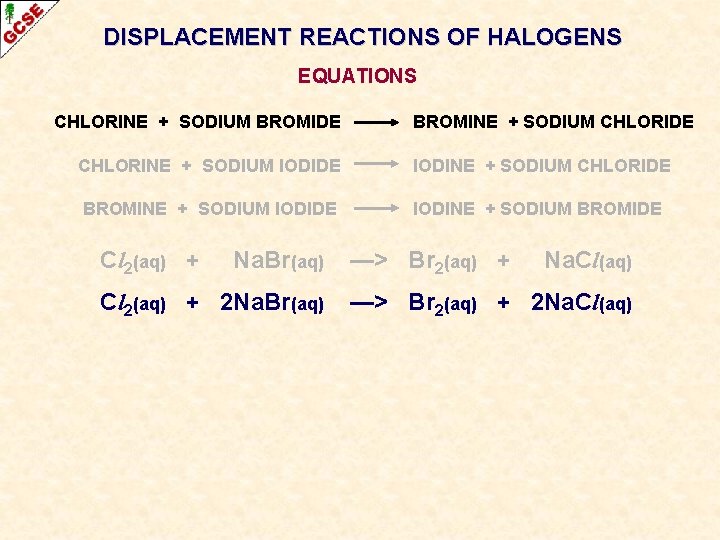
DISPLACEMENT REACTIONS OF HALOGENS EQUATIONS CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM IODIDE IODINE + SODIUM CHLORIDE BROMINE + SODIUM IODIDE IODINE + SODIUM BROMIDE Cl 2(aq) + Na. Br(aq) —> Br 2(aq) + Na. Cl(aq) The equation doesn’t balance - multiply the formulae until it does

DISPLACEMENT REACTIONS OF HALOGENS EQUATIONS CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM IODIDE IODINE + SODIUM CHLORIDE BROMINE + SODIUM IODIDE IODINE + SODIUM BROMIDE Cl 2(aq) + Na. Br(aq) Cl 2(aq) + 2 Na. Br(aq) —> Br 2(aq) + Na. Cl(aq) —> Br 2(aq) + 2 Na. Cl(aq)

DISPLACEMENT REACTIONS OF HALOGENS EQUATIONS CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM IODIDE IODINE + SODIUM CHLORIDE BROMINE + SODIUM IODIDE IODINE + SODIUM BROMIDE Cl 2(aq) + 2 Na. Br(aq) —> Br 2(aq) + 2 Na. Cl(aq) IONIC EQUATION Cl 2(aq) + 2 Br¯(aq) —> Br 2(aq) + 2 Cl¯(aq)

DISPLACEMENT REACTIONS OF HALOGENS EQUATIONS CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM IODIDE IODINE + SODIUM CHLORIDE BROMINE + SODIUM IODIDE IODINE + SODIUM BROMIDE Cl 2(aq) + 2 Na. I(aq) —> I 2(aq) + 2 Na. Cl(aq) IONIC EQUATION Cl 2(aq) + 2 I¯(aq) —> I 2(aq) + 2 Cl¯(aq)

DISPLACEMENT REACTIONS OF HALOGENS EQUATIONS CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM IODIDE IODINE + SODIUM CHLORIDE BROMINE + SODIUM IODIDE IODINE + SODIUM BROMIDE Br 2(aq) + 2 Na. I(aq) —> I 2(aq) + 2 Na. Br(aq) IONIC EQUATION Br 2(aq) + 2 I¯(aq) —> I 2(aq) + 2 Br¯(aq)

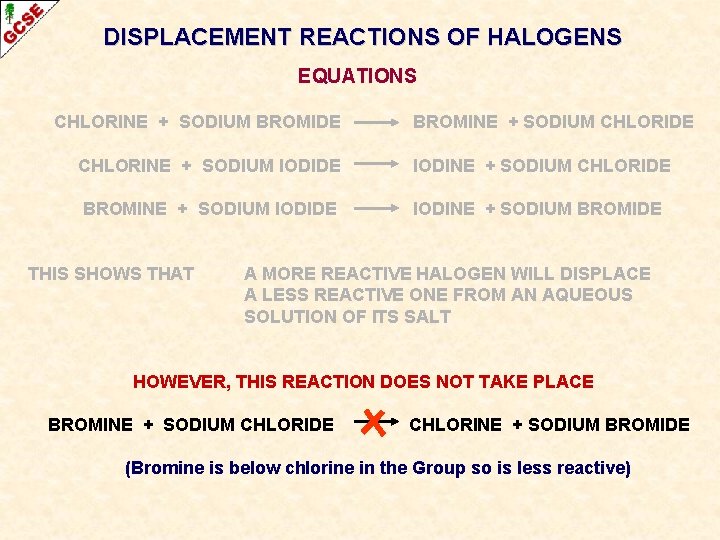
DISPLACEMENT REACTIONS OF HALOGENS SUMMARY CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM IODIDE IODINE + SODIUM CHLORIDE BROMINE + SODIUM IODIDE IODINE + SODIUM BROMIDE THIS SHOWS THAT A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE FROM AN AQUEOUS SOLUTION OF ITS SALT

DISPLACEMENT REACTIONS OF HALOGENS EQUATIONS CHLORINE + SODIUM BROMIDE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM IODIDE IODINE + SODIUM CHLORIDE BROMINE + SODIUM IODIDE IODINE + SODIUM BROMIDE THIS SHOWS THAT A MORE REACTIVE HALOGEN WILL DISPLACE A LESS REACTIVE ONE FROM AN AQUEOUS SOLUTION OF ITS SALT HOWEVER, THIS REACTION DOES NOT TAKE PLACE BROMINE + SODIUM CHLORIDE CHLORINE + SODIUM BROMIDE (Bromine is below chlorine in the Group so is less reactive)

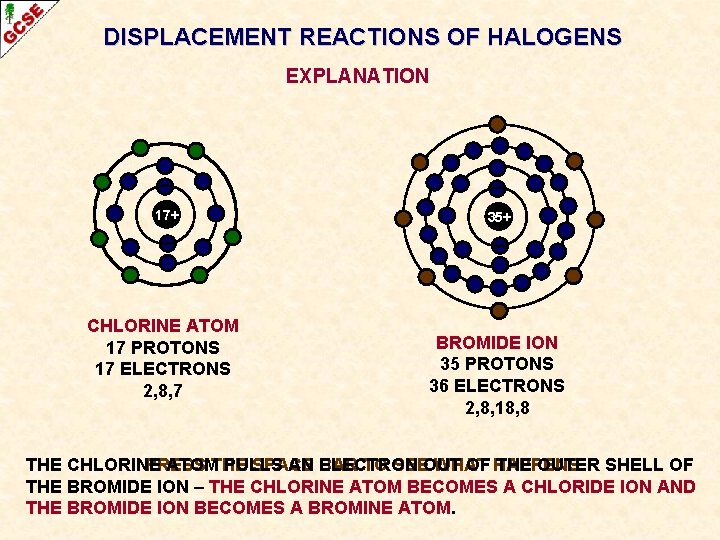
DISPLACEMENT REACTIONS OF HALOGENS EXPLANATION 17+ CHLORINE ATOM 17 PROTONS 17 ELECTRONS 2, 8, 7 35+ BROMIDE ION 35 PROTONS 36 ELECTRONS 2, 8, 18, 8 THE CHLORINE PRESS ATOMTHE PULLS SPACE AN BAR ELECTRON TO SEEOUT WHAT OF HAPPENS THE OUTER SHELL OF THE BROMIDE ION – THE CHLORINE ATOM BECOMES A CHLORIDE ION AND THE BROMIDE ION BECOMES A BROMINE ATOM.

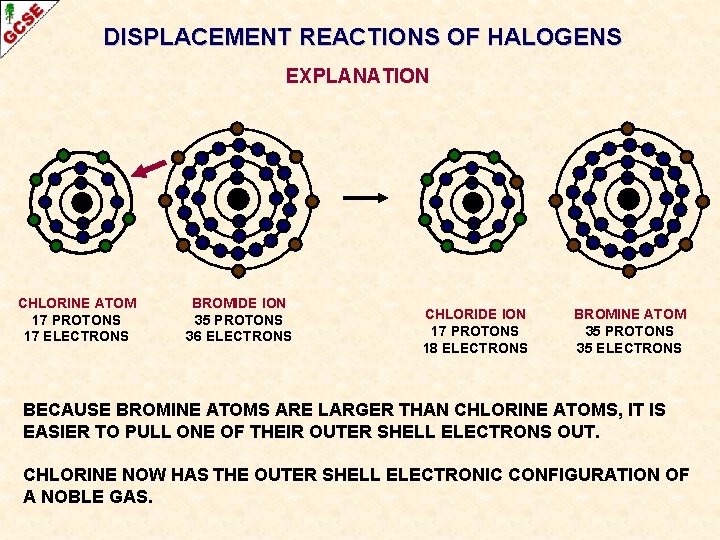
DISPLACEMENT REACTIONS OF HALOGENS EXPLANATION CHLORINE ATOM 17 PROTONS 17 ELECTRONS BROMIDE ION 35 PROTONS 36 ELECTRONS CHLORIDE ION 17 PROTONS 18 ELECTRONS BROMINE ATOM 35 PROTONS 35 ELECTRONS BECAUSE BROMINE ATOMS ARE LARGER THAN CHLORINE ATOMS, IT IS EASIER TO PULL ONE OF THEIR OUTER SHELL ELECTRONS OUT. CHLORINE NOW HAS THE OUTER SHELL ELECTRONIC CONFIGURATION OF A NOBLE GAS.

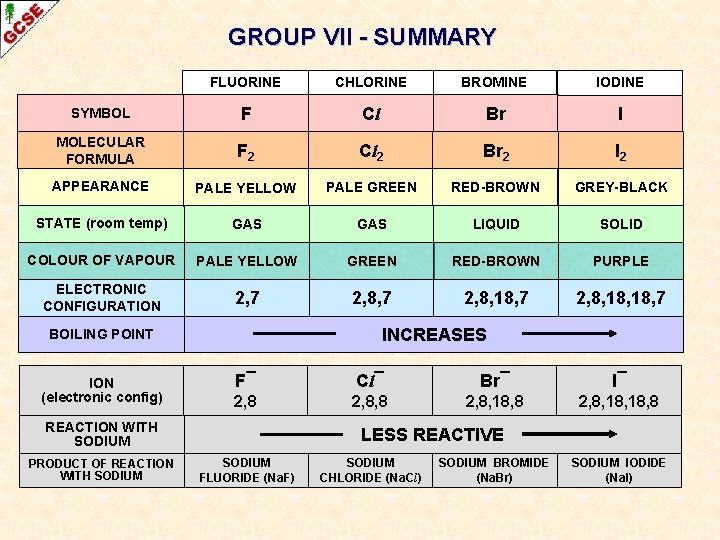
GROUP VII - SUMMARY FLUORINE CHLORINE BROMINE IODINE SYMBOL F Cl Br I MOLECULAR FORMULA F 2 Cl 2 Br 2 I 2 APPEARANCE PALE YELLOW PALE GREEN RED-BROWN GREY-BLACK STATE (room temp) GAS LIQUID SOLID COLOUR OF VAPOUR PALE YELLOW GREEN RED-BROWN PURPLE ELECTRONIC CONFIGURATION 2, 7 2, 8, 18, 7 INCREASES BOILING POINT ION (electronic config) F¯ Cl¯ Br¯ I¯ 2, 8, 8 2, 8, 18, 18, 8 REACTION WITH SODIUM PRODUCT OF REACTION WITH SODIUM LESS REACTIVE SODIUM FLUORIDE (Na. F) SODIUM CHLORIDE (Na. Cl) SODIUM BROMIDE (Na. Br) SODIUM IODIDE (Na. I)

QUICK QUIZ 1. ELEMENTS IN GROUP 7 ARE KNOWN AS THE ……… 2. WHAT ARE THE NAMES OF THE ELEMENTS 3. HOW DOES THE ATOMIC NUMBER CHANGE DOWN THE GROUP? 4. HOW DOES THE ELECTRONIC CONFIGURATION CHANGE? 5. HOW DOES THE ATOMIC SIZE (RADIUS) CHANGE? 6. HOW MANY ELECTRONS DO THEY HAVE IN THE OUTER LEVEL? 7. ARE THEY METALS OR NON-METALS? 8. WHAT HAPPENS TO THEIR COLOUR DOWN THE GROUP? 9. DO THEY GO AROUND IN PAIRS OR AS MONATOMIC GASES? 10. WHAT HAPPENS TO THEIR STATE AT ROOM TEMPERATURE? 11. WHAT TYPE OF COMPOUNDS DO THEY FORM WITH METALS? 12. HOW CAN EXPLAIN THEIR RELATIVE REACTIVITY IN TERMS OF THE ATOMIC STRUCTURE?

QUICK QUIZ - ANSWERS 1. HALOGENS. 2. FLUORINE, CHLORINE, BROMINE, IODINE, ASTATINE. 3. ATOMIC NUMBER INCREASES DOWN THE GROUP. 4. GET MORE SHELLS DOWN THE GROUP. 5. ATOMIC SIZE INCREASES DOWN THE GROUP. 6. THEY ALL HAVE SEVEN ELECTRONS IN THE OUTER LEVEL. 7. THEY ARE NON-METALS. 8. COLOUR DARKENS DOWN THE GROUP. 9. ATOMS GO AROUND IN PAIRS OR AS DIATOMIC GASES. 10. GO FROM GAS TO SOLID DOWN THE GROUP. 11. THEY FORM IONIC COMPOUNDS WITH METALS. 12. THE LARGER THEY ARE THE LESS EASILY ELECTRONS ARE GAINED AND THE LESS REACTIVE THEY BECOME.

GROUP VII The Halogens THE END © 2011 KNOCKHARDY PUBLISHING & JONATHAN HOPTON