Group Quizzes All group quizzes and answers for















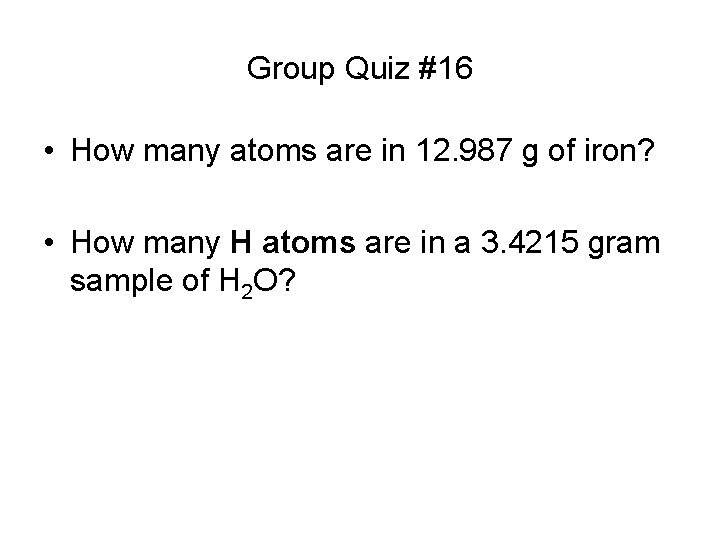


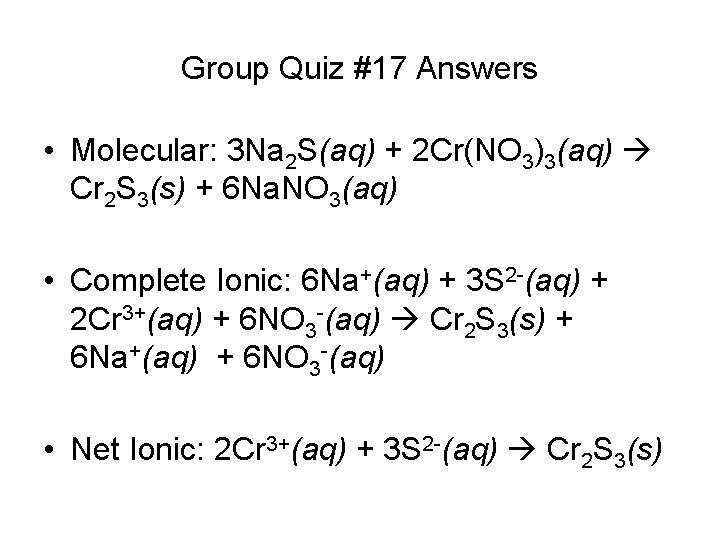












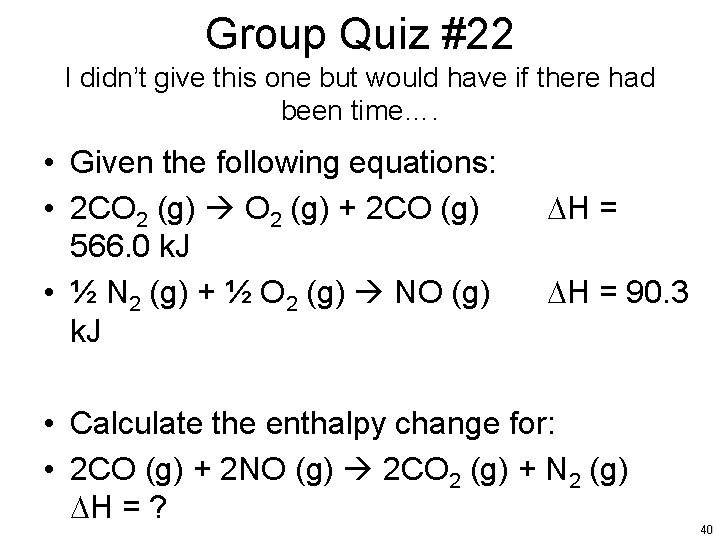



- Slides: 43

Group Quizzes All group quizzes and answers for this semester! The numbers are off – deal with it.

Group Quiz 1 • Logging in to Mastering. Chemistry

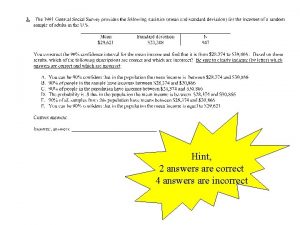
Syllabus quiz: Group Quiz #2 • How many hour exams are there? How many get dropped? • What happens if you miss an exam? • How many points is the final exam worth? • How much is each homework assignment worth? • How many points is each group quiz worth and how many will count toward your final grade? • When are my office hours? • Name the author(s) of the textbook we are using this semester. • What sources of help are available to help you succeed in this course (list at least 3)?

Dimensional Analysis Practice • Problem 1. 17: Gemstones are weighed in carats, with 1 carat = 200 mg (exactly). What is the mass, in grams, of the Hope Diamond, the world’s largest blue diamond at 44. 4 carats? • The density of a steel ball bearing is 7. 81 g/cm 3. If the ball bearing is measured to have a volume of 1. 34 cm 3, what is its mass in milligrams?

Group Quiz #3 • Dimensional Analysis: One lap in an olympic swimming pool is 50 meters exactly. If an athlete swims 80 laps in one practice, how far did he/she swim (in miles)? • 1 inch = 2. 54 cm (exactly) • 12 inches = 1 foot (exactly) • 1 mile = 5280 feet (exactly)

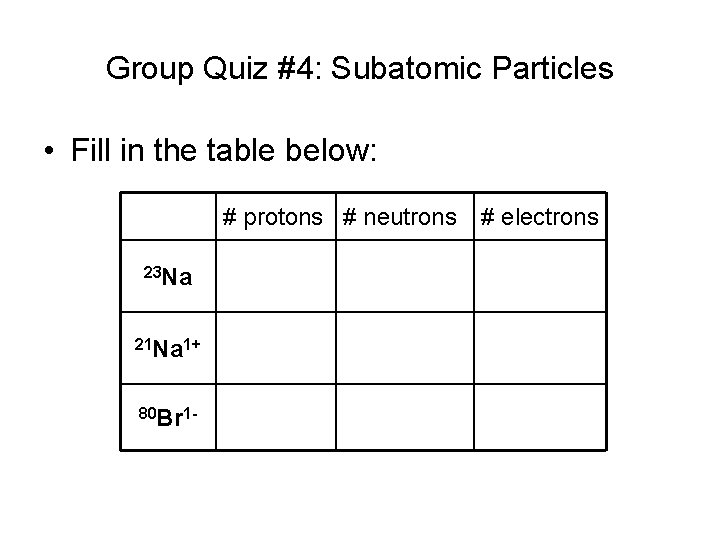
Group Quiz #4: Subatomic Particles • Fill in the table below: # protons # neutrons 23 Na 21 Na 1+ 80 Br 1 - # electrons

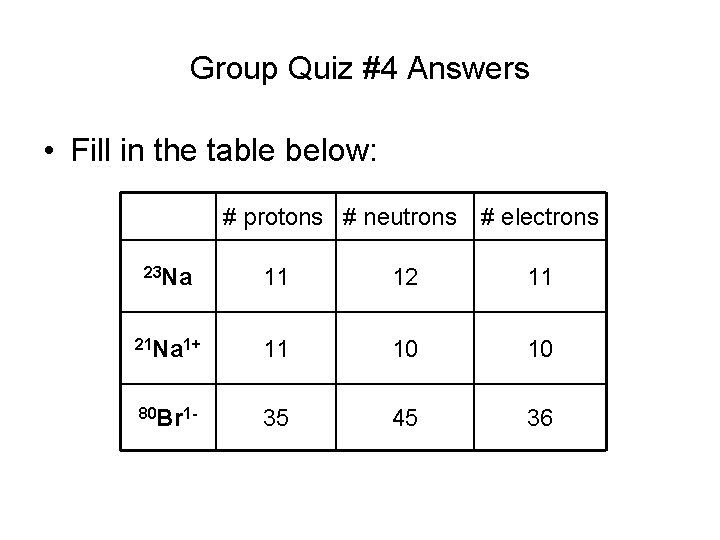
Group Quiz #4 Answers • Fill in the table below: # protons # neutrons # electrons 23 Na 11 12 11 21 Na 1+ 11 10 10 80 Br 1 - 35 45 36

Group Quiz #5 • Calculate the energy of a photon that has a wavelength of 35. 6 nm (in the x-ray region). (Hint: Watch units!!!)

Group Quiz #5 Answer • Wavelength: 3. 56 x 10 -8 m • Frequency: 8. 43 x 1015 s-1 • Energy: 5. 58 x 10 -18 J

Group Quiz #6 • Write electron configurations for the following atoms/ions. • K (long-hand) • S 2 - (long-hand) • Mo (short-hand) • Al 3+ (long-hand) • Fe 2+ (short-hand) 10

Group Quiz #6 Answers • Write electron configurations for the following ions. • K (long-hand): 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 • S 2 - (long-hand): 1 s 2 2 p 6 3 s 2 3 p 6 • Mo (short-hand): [Kr] 5 s 1 4 d 5 • Al 3+ (long-hand): 1 s 2 2 p 6 • Fe 2+ (short-hand): [Ar] 4 s 0 3 d 6 11

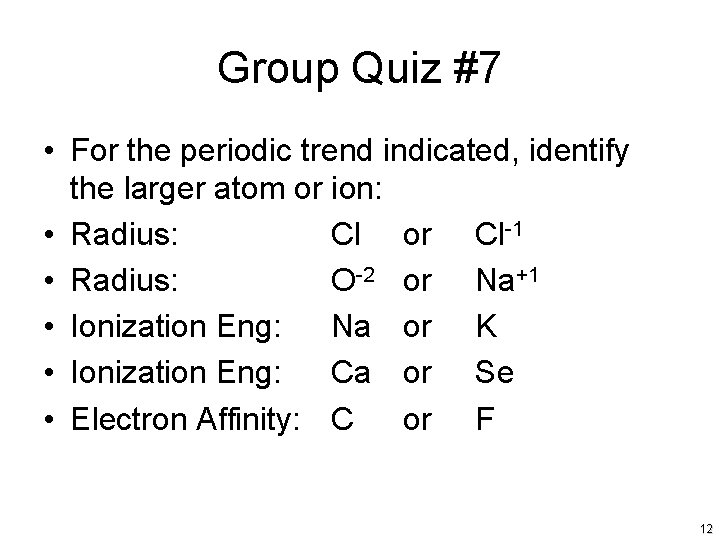
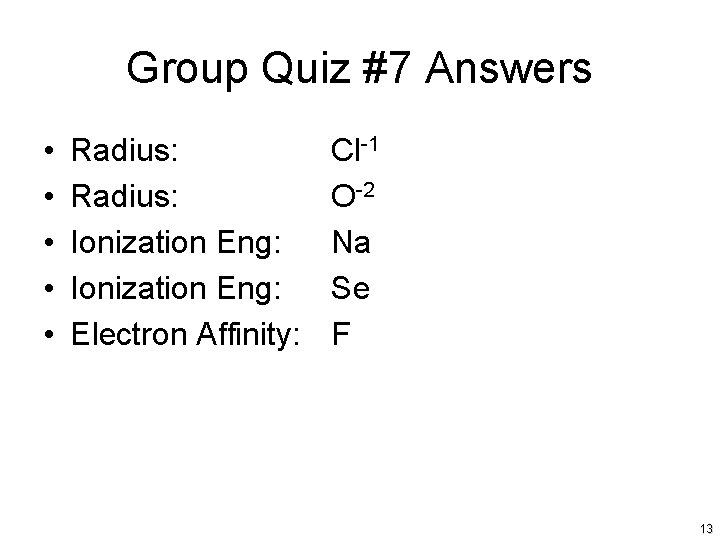
Group Quiz #7 • For the periodic trend indicated, identify the larger atom or ion: • Radius: Cl or Cl-1 • Radius: O-2 or Na+1 • Ionization Eng: Na or K • Ionization Eng: Ca or Se • Electron Affinity: C or F 12

Group Quiz #7 Answers • • • Radius: Ionization Eng: Electron Affinity: Cl-1 O-2 Na Se F 13

Group Quiz #8 • Write names formulas and formulas for names: • • • Xe. F 6 ammonium sulfite Cr 3 N 2 K 3 PO 4 hydrochloric acid 14

Group Quiz #8 Answers • • • Name the following compounds: Xe. F 6: xenon hexafluoride ammonium sulfite: (NH 4)2 SO 3 Cr 3 N 2: chromium (II) nitride K 3 PO 4: potassium phosphate hydrochloric acid: HCl (aq) 15

Group Quiz #11 Sorry: No answers for these…. • Draw all possible Lewis Structures for: • • • POCl 3 BF 3 NO 2 PF 5 SO 3

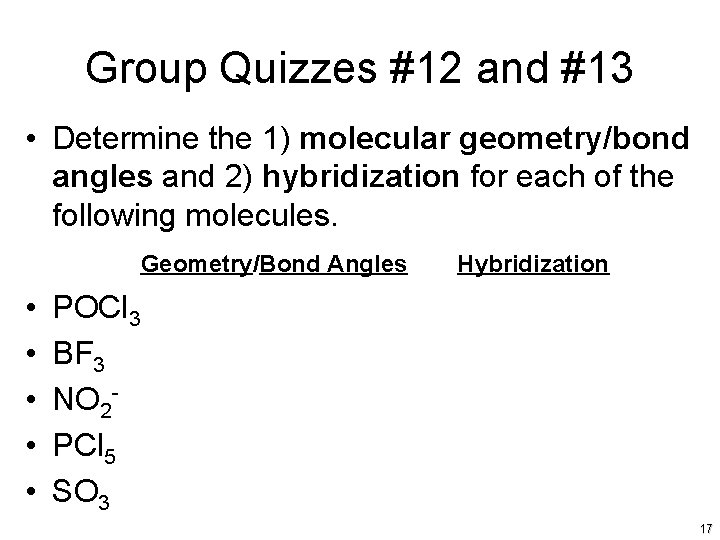
Group Quizzes #12 and #13 • Determine the 1) molecular geometry/bond angles and 2) hybridization for each of the following molecules. Geometry/Bond Angles • • • Hybridization POCl 3 BF 3 NO 2 PCl 5 SO 3 17

Group Quiz #12 and #13 Answers • • • Tetrahedral, 109. 5 o, sp 3 Trigonal Planar, 120 o, sp 2 Bent, <120 o, sp 2 Trigonal Bipyramidal, 120 o and 90 o, sp 3 d Trigonal Planar, 120 o, sp 2 18

Group Quiz #14 • Which member of each pair has stronger intermolecular force (boiling point, heat of vaporization)? Explain your reasoning. CH 4 or CH 3 NH 3 or NF 3 CO 2 or SO 2 or O 3 I 2 or Cl 2 19

Group Quiz #14 Answers • Which member of each pair has stronger intermolecular force (boiling point, heat of vaporization)? Explain your reasoning. CH 3 larger NH 3 H-bridging SO 2 dipole-dipole O 3 dipole-dipole I 2 larger

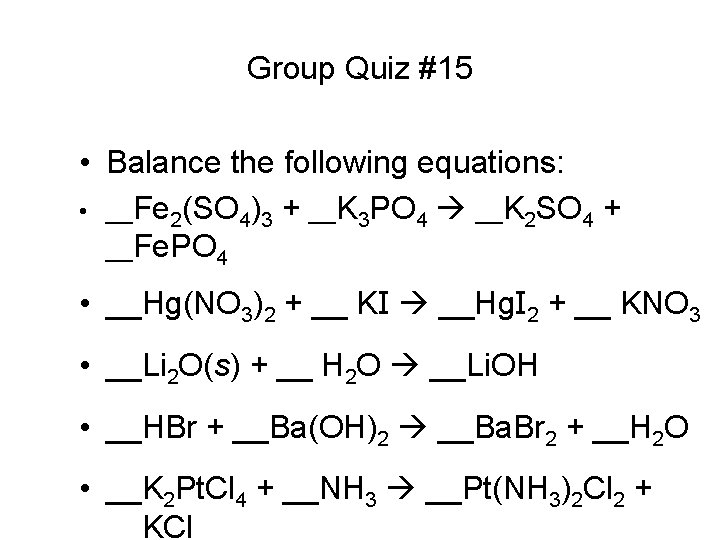
Group Quiz #15 • Balance the following equations: • __Fe 2(SO 4)3 + __K 3 PO 4 __K 2 SO 4 + __Fe. PO 4 • __Hg(NO 3)2 + __ KI __Hg. I 2 + __ KNO 3 • __Li 2 O(s) + __ H 2 O __Li. OH • __HBr + __Ba(OH)2 __Ba. Br 2 + __H 2 O • __K 2 Pt. Cl 4 + __NH 3 __Pt(NH 3)2 Cl 2 + __KCl

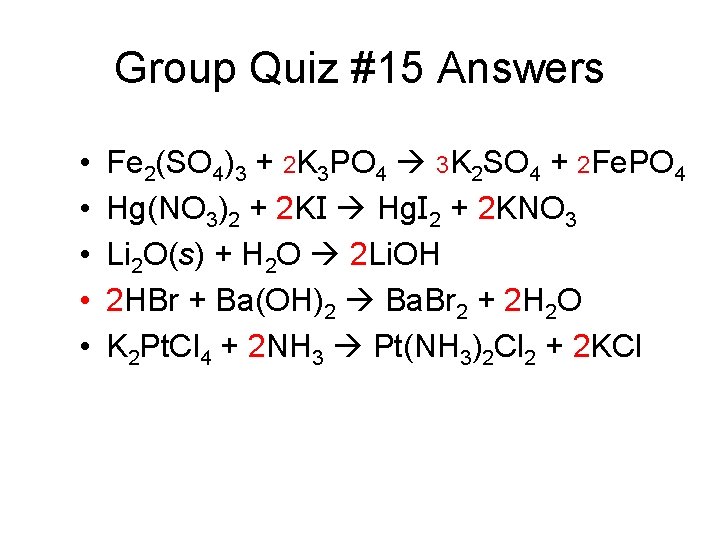
Group Quiz #15 Answers • • • Fe 2(SO 4)3 + 2 K 3 PO 4 3 K 2 SO 4 + 2 Fe. PO 4 Hg(NO 3)2 + 2 KI Hg. I 2 + 2 KNO 3 Li 2 O(s) + H 2 O 2 Li. OH 2 HBr + Ba(OH)2 Ba. Br 2 + 2 H 2 O K 2 Pt. Cl 4 + 2 NH 3 Pt(NH 3)2 Cl 2 + 2 KCl
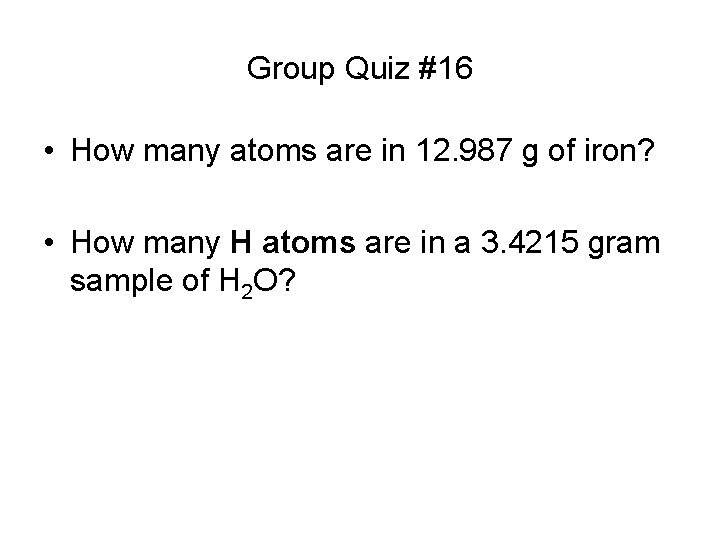
Group Quiz #16 • How many atoms are in 12. 987 g of iron? • How many H atoms are in a 3. 4215 gram sample of H 2 O?

Group Quiz #16 Answers • How many moles are in 12. 987 g of iron? • How many H atoms are in a 3. 4215 gram sample of H 2 O?

Group Quiz #17 • Determine the products of the reaction. Identify the phase of each compound, and balance the equation. Also write the ionic and net ionic equations. • Molecular: Na 2 S + Cr(NO 3)3 • Complete Ionic: • Net Ionic:
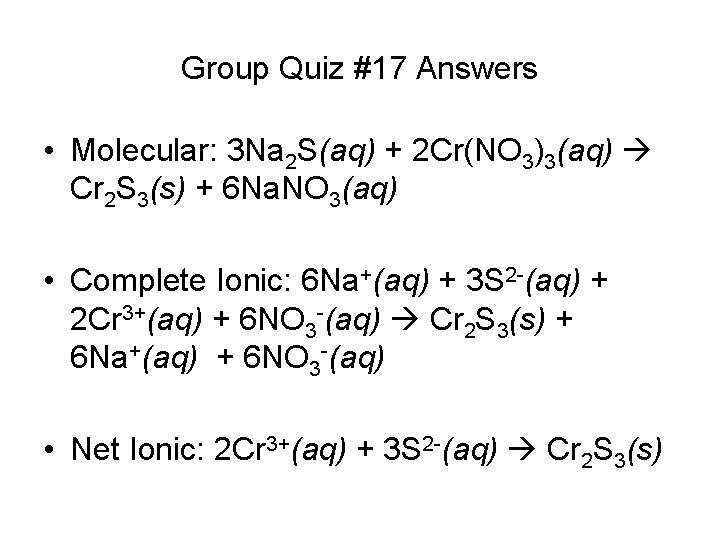
Group Quiz #17 Answers • Molecular: 3 Na 2 S(aq) + 2 Cr(NO 3)3(aq) Cr 2 S 3(s) + 6 Na. NO 3(aq) • Complete Ionic: 6 Na+(aq) + 3 S 2 -(aq) + 2 Cr 3+(aq) + 6 NO 3 -(aq) Cr 2 S 3(s) + 6 Na+(aq) + 6 NO 3 -(aq) • Net Ionic: 2 Cr 3+(aq) + 3 S 2 -(aq) Cr 2 S 3(s)

Group Quiz #15 • Identify the oxidation number of each element in the compounds or ions below: • Ba(Cl. O 3)2 • SO 32 -

Group Quiz #15 Answers • Ba: +2 • Cl: +5 • O: -2 • S: +4 • O: -2

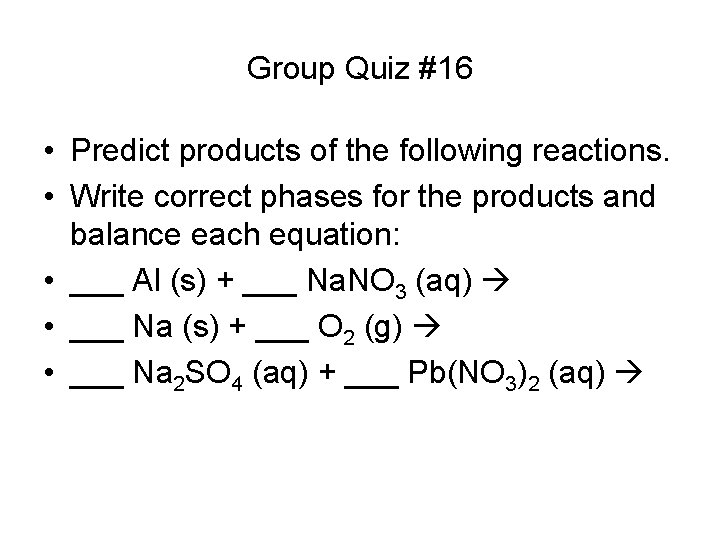
Group Quiz #16 • Predict products of the following reactions. • Write correct phases for the products and balance each equation: • ___ Al (s) + ___ Na. NO 3 (aq) • ___ Na (s) + ___ O 2 (g) • ___ Na 2 SO 4 (aq) + ___ Pb(NO 3)2 (aq)

Group Quiz #16 Answers • Al (s) + Na. NO 3 (aq) No reaction • 4 Na (s) + O 2 (g) 2 Na 2 O (s) • Na 2 SO 4 (aq) + Pb(NO 3)2 (aq) 2 Na. NO 3 (aq) + Pb. SO 4 (s)

Group Quiz #17 • Complete the equation below (with phases) and balance it. • K 2 S(aq) + Ag. NO 3(g) • If you combine 10. 21 m. L of 0. 152 M K 2 S with 1. 0092 g Ag. NO 3, how much solid product can be formed? • If 0. 5744 g of product were actually formed, what is the percent yield?

Group Quiz #17 Answers • Complete the equation below (with phases) and balance it. – K 2 S(aq) + 2 Ag. NO 3(aq) Ag 2 S(s) + 2 KNO 3(aq) • If 0. 5744 g of product were actually formed, what is the percent yield?

Group Quiz #19 • What is the concentration of sodium hydroxide if it took 23. 48 m. L of base to neutralize 15. 39 m. L of 1. 20 M phosporic acid?

Group Quiz #19 Answer

Group Quiz #18 • What mass of sodium acetate must be used to make 100. 0 m. L of a 0. 100 M solution? • What is the concentration of this solution if you then add 400. 0 m. L of water to it?

Group Quiz #18 Answers • What mass of sodium acetate must be used to make 100. 0 m. L of a 0. 100 M solution? • What is the concentration of this solution if you then add another 400. 0 m. L of water to it?

Group Quiz #19 Sorry: No answers…. • What mass of sodium azide (Na. N 3) is needed to generate 75. 2 L of nitrogen gas (and solid sodium) at 100. 0 o. C and 1. 00 atm? • (Hint: Start with a balanced chemical equation!)

Group Quiz #21 • A hot piece of copper (at 98. 7 o. C, specific heat = 0. 385 J/g • o. C) weighs 34. 6486 g. When placed in room temperature water, it is calculated that 915. 1 J of heat are released by the metal. • What gains heat? What loses heat? What is the final temperature of the metal? • Watch signs!!!!

Group Quiz #21 Answer • -951. 1 J = (34. 6486 g)(0. 385 J/g • o. C)(x – 98. 7 o. C) • -68. 5997 o. C = x – 98. 7 o. C • x = 30. 1 o. C
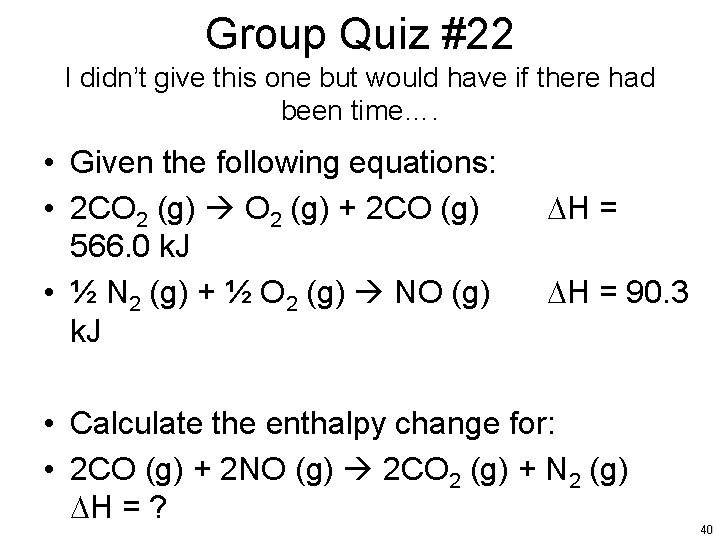
Group Quiz #22 I didn’t give this one but would have if there had been time…. • Given the following equations: • 2 CO 2 (g) + 2 CO (g) 566. 0 k. J • ½ N 2 (g) + ½ O 2 (g) NO (g) k. J DH = 90. 3 • Calculate the enthalpy change for: • 2 CO (g) + 2 NO (g) 2 CO 2 (g) + N 2 (g) DH = ? 40

Group Quiz #22 Answers • 1: Reverse • O 2 (g) + 2 CO (g) 2 CO 2 (g) DH = -566. 0 k. J • 2: Reverse, x 2 • 2 NO (g) N 2 (g) + O 2 (g) DH = -180. 6 k. J • 2 CO (g) + 2 NO (g) 2 CO 2 (g) + N 2 (g) =DH = -746. 6 k. J

Group Quiz #22 – Given in class • Use Standard Heat of Formation values to calculate the enthalpy of reaction for: • C 6 H 12 O 6(s) C 2 H 5 OH(l) + CO 2(g) • Hint: Is the equation balanced? DHof (C 6 H 12 O 6(s)) = -1260. 0 k. J/mol DHof (C 2 H 5 OH(l)) = -277. 7 k. J/mol DHof (CO 2(g)) = -393. 5 k. J/mol

Group Quiz #22 Answer • C 6 H 12 O 6(s) 2 C 2 H 5 OH(l) + 2 CO 2(g) • DHof (C 6 H 12 O 6(s)) = -1260. 0 k. J/mol • DHof (C 2 H 5 OH(l)) = -277. 7 k. J/mol • DHof (CO 2(g)) = -393. 5 k. J/mol • DHorxn = [2 mol (-277. 7 k. J/mol) + 2 mol (-393. 5 k. J/mol)] – [1 mol (-1260. 0 k. J/mol)] = -82. 4 k. J
 Name all the lines name all the segments name all the rays
Name all the lines name all the segments name all the rays Jlab quizzes
Jlab quizzes Mpi questions and answers
Mpi questions and answers Fft quizzes
Fft quizzes Night by elie wiesel quizzes
Night by elie wiesel quizzes Quizzes
Quizzes Are you dirty minded quiz
Are you dirty minded quiz Https://www.tate.org.uk/kids/games-quizzes/street-art
Https://www.tate.org.uk/kids/games-quizzes/street-art 8 types of energy
8 types of energy Quizzes
Quizzes Weighfair
Weighfair Frankenstein letters quiz
Frankenstein letters quiz Quizzes for students
Quizzes for students Holes questions chapter 1-5
Holes questions chapter 1-5 Quizzes
Quizzes Quizzes
Quizzes Sparc quiz answers
Sparc quiz answers Vle itsligo
Vle itsligo Quizzes
Quizzes Quizzes
Quizzes Quizzes
Quizzes The odyssey book 1 quiz
The odyssey book 1 quiz Happy monday quiz
Happy monday quiz Phys 241 purdue
Phys 241 purdue Fractions amp; decimals quick quizzes ages 7 9 download
Fractions amp; decimals quick quizzes ages 7 9 download Quizzes
Quizzes Quizzes
Quizzes Introduction to java programming 10th edition quizzes
Introduction to java programming 10th edition quizzes Kontinuitetshantering
Kontinuitetshantering Typiska novell drag
Typiska novell drag Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Underlag för särskild löneskatt på pensionskostnader
Underlag för särskild löneskatt på pensionskostnader Tidböcker
Tidböcker Anatomi organ reproduksi
Anatomi organ reproduksi Vad är densitet
Vad är densitet Datorkunskap för nybörjare
Datorkunskap för nybörjare Stig kerman
Stig kerman Debattinlägg mall
Debattinlägg mall För och nackdelar med firo
För och nackdelar med firo Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon