GROUP 2 PHYSICAL AND CHEMICAL PROPERTIES AND USES







- Slides: 34

GROUP 2 – PHYSICAL AND CHEMICAL PROPERTIES AND USES

GROUP II Learning Objectives Learners will: 1) know and be able to explain the uses of Ca(OH)2 2) understand be able to account for trends in melting point, atomic radius, 1 st ionisation energy, electronegativity 3) know and be able to account for the trends in reactivity with water, the relative solubility of the hydroxides and sulfates 4) know and be able to explain the uses of Mg(OH)2 and Ca(OH)2 5) know the use of Ba. Cl 2 solution to test for sulfates and the use of Ba. SO 4 in medicine

Fuse School (Group 2) https: //www. youtube. com/watch? v=yk. ZYZdl 8 Fe. I

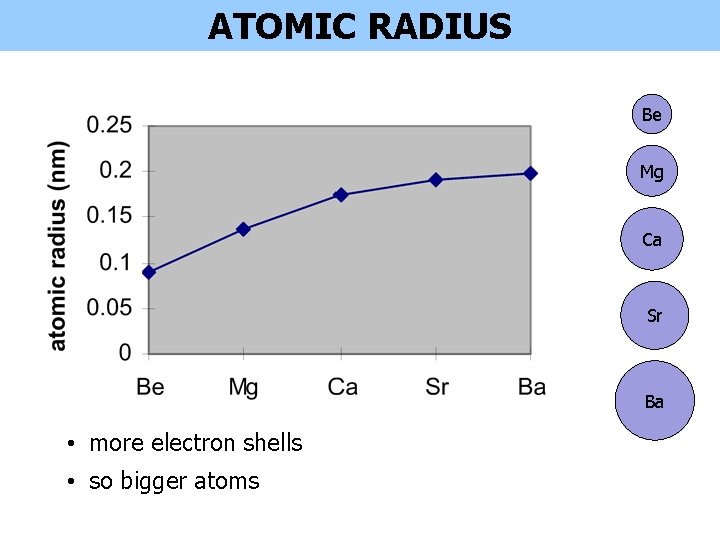
ATOMIC RADIUS Be Mg Ca Sr Ba • more electron shells • so bigger atoms

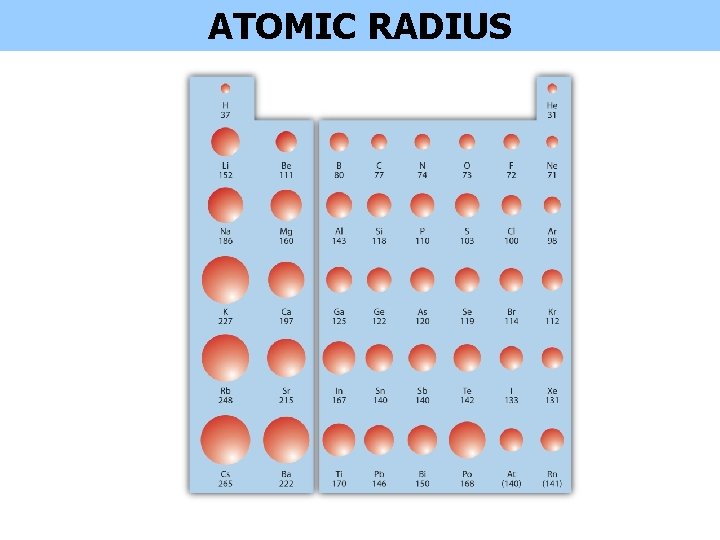
ATOMIC RADIUS

Why do Radii decrease across a Period Atomic size gradually decreases from left to right across a period of elements. This is because, within a period all electrons are added to the same shell. At the same time, protons are being added to the nucleus. BUT we are not adding distance. This means that the nucleus attracts the electrons more strongly, pulling the atom's shell closer to the nucleus. The valence electrons are held closer towards the nucleus of the atom.

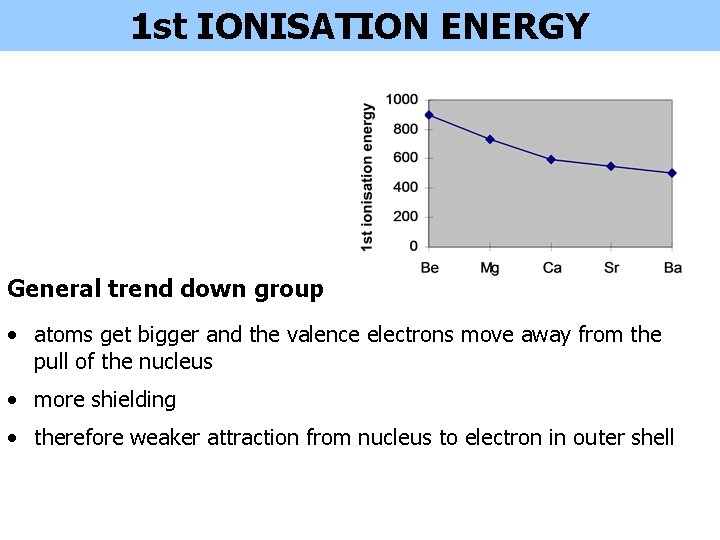
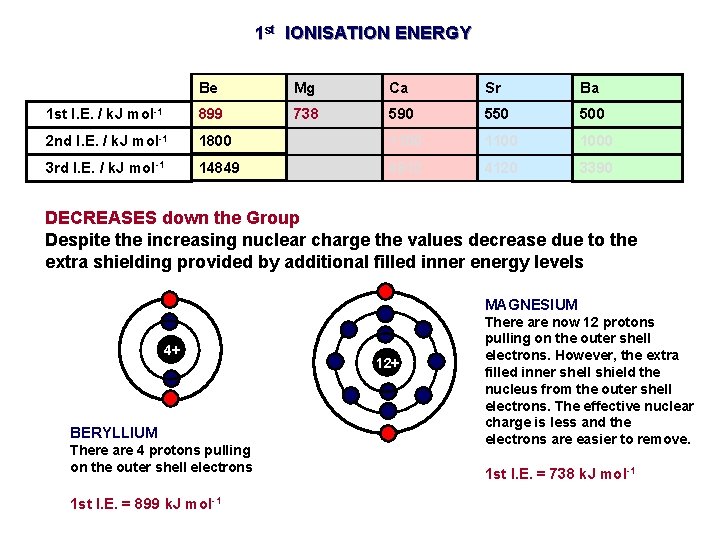
1 st IONISATION ENERGY General trend down group • atoms get bigger and the valence electrons move away from the pull of the nucleus • more shielding • therefore weaker attraction from nucleus to electron in outer shell

1 st IONISATION ENERGY Be Mg Ca Sr Ba 1 st I. E. / k. J mol-1 899 738 590 550 500 2 nd I. E. / k. J mol-1 1800 1500 1100 1000 3 rd I. E. / k. J mol-1 14849 7733 4912 4120 3390 DECREASES down the Group Despite the increasing nuclear charge the values decrease due to the extra shielding provided by additional filled inner energy levels MAGNESIUM 4+ BERYLLIUM There are 4 protons pulling on the outer shell electrons 1 st I. E. = 899 k. J mol-1 12+ There are now 12 protons pulling on the outer shell electrons. However, the extra filled inner shell shield the nucleus from the outer shell electrons. The effective nuclear charge is less and the electrons are easier to remove. 1 st I. E. = 738 k. J mol-1

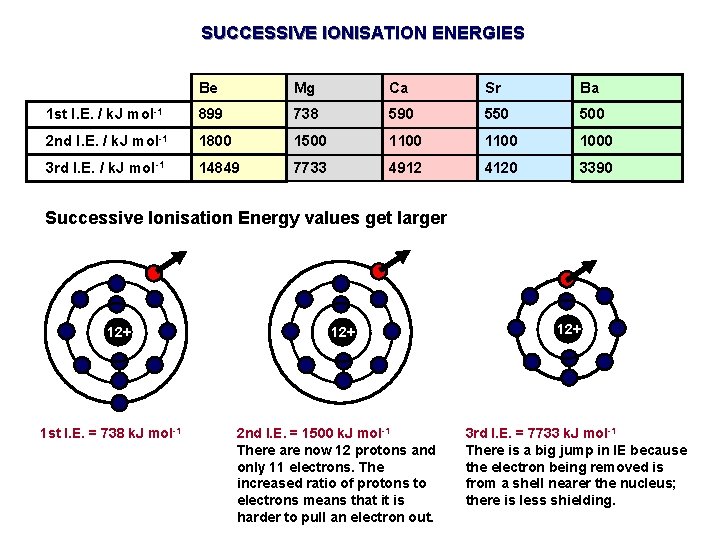
SUCCESSIVE IONISATION ENERGIES Be Mg Ca Sr Ba 1 st I. E. / k. J mol-1 899 738 590 550 500 2 nd I. E. / k. J mol-1 1800 1500 1100 1000 3 rd I. E. / k. J mol-1 14849 7733 4912 4120 3390 Successive Ionisation Energy values get larger 12+ 1 st I. E. = 738 k. J mol-1 12+ 2 nd I. E. = 1500 k. J mol-1 There are now 12 protons and only 11 electrons. The increased ratio of protons to electrons means that it is harder to pull an electron out. 12+ 3 rd I. E. = 7733 k. J mol-1 There is a big jump in IE because the electron being removed is from a shell nearer the nucleus; there is less shielding.

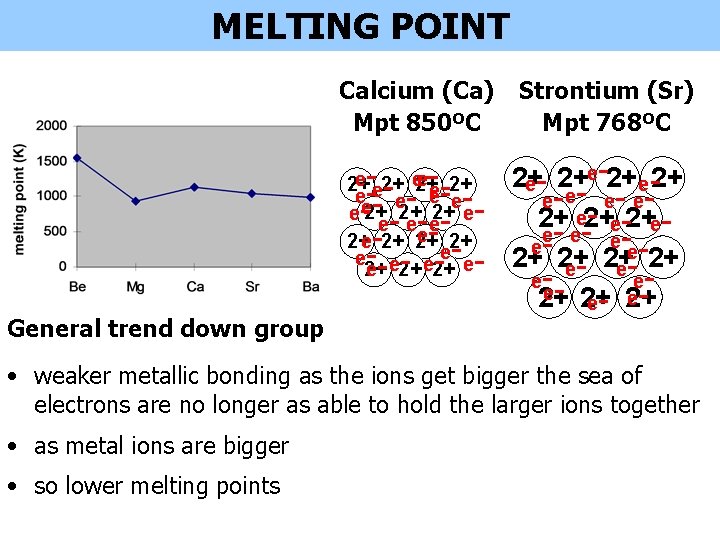
MELTING POINT Calcium (Ca) Mpt 850ºC Strontium (Sr) Mpt 768ºC e- - --2+ e- 2+ 2+e-2+ -- - e- e- - -- - e-2+e 2+ e- e- e- -ee-2+ -- - 2+ 2+ 2+ e- eeee e 2+ 2+ e- 2+ e 2+ 2+ e e e e 2+ 2+ 2+ e e ee e 2+ 2+ General trend down group • weaker metallic bonding as the ions get bigger the sea of electrons are no longer as able to hold the larger ions together • as metal ions are bigger • so lower melting points

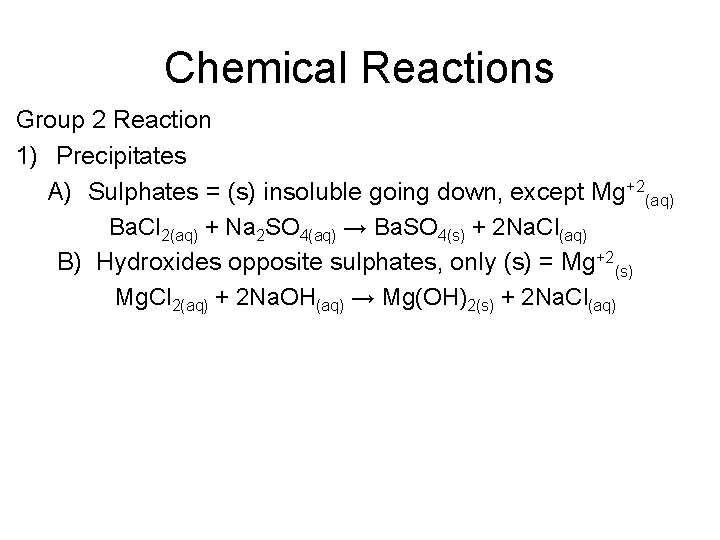
Chemical Reactions Group 2 Reaction 1) Precipitates A) Sulphates = (s) insoluble going down, except Mg+2(aq) Ba. Cl 2(aq) + Na 2 SO 4(aq) → Ba. SO 4(s) + 2 Na. Cl(aq) B) Hydroxides opposite sulphates, only (s) = Mg+2(s) Mg. Cl 2(aq) + 2 Na. OH(aq) → Mg(OH)2(s) + 2 Na. Cl(aq)

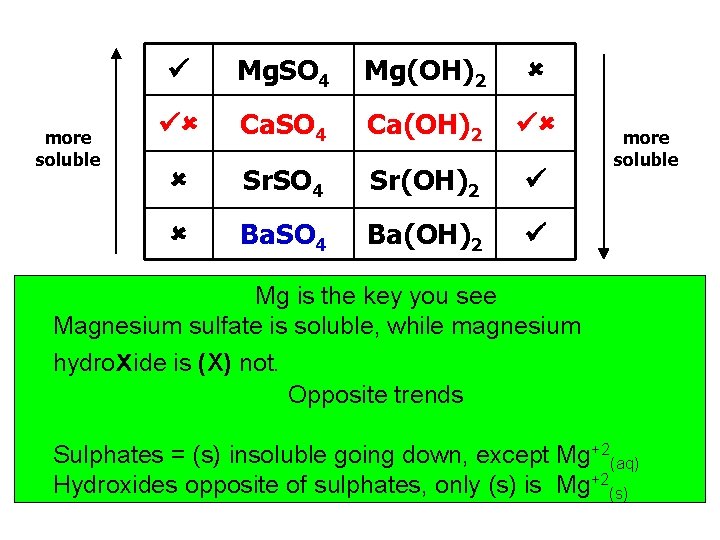
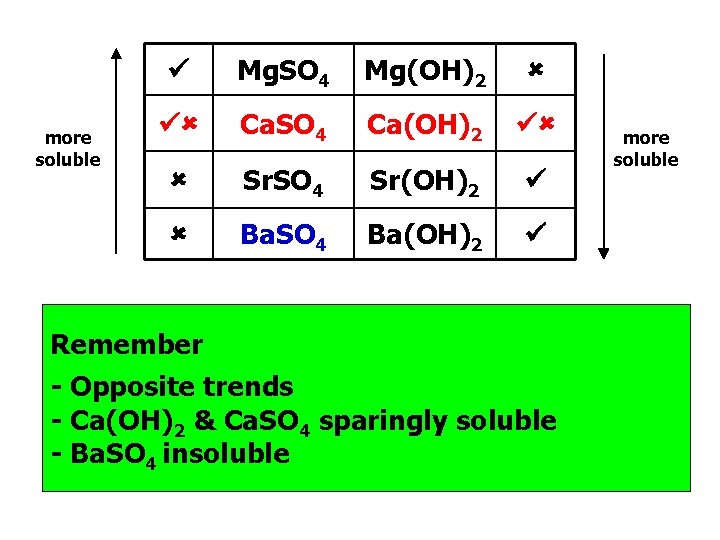
more soluble Mg. SO 4 Mg(OH)2 Ca. SO 4 Ca(OH)2 Sr. SO 4 Sr(OH)2 Ba. SO 4 Ba(OH)2 more soluble Mg is the key you see Magnesium sulfate is soluble, while magnesium hydroxide is (X) not. Opposite trends Sulphates = (s) insoluble going down, except Mg+2(aq) Hydroxides opposite of sulphates, only (s) is Mg+2(s)

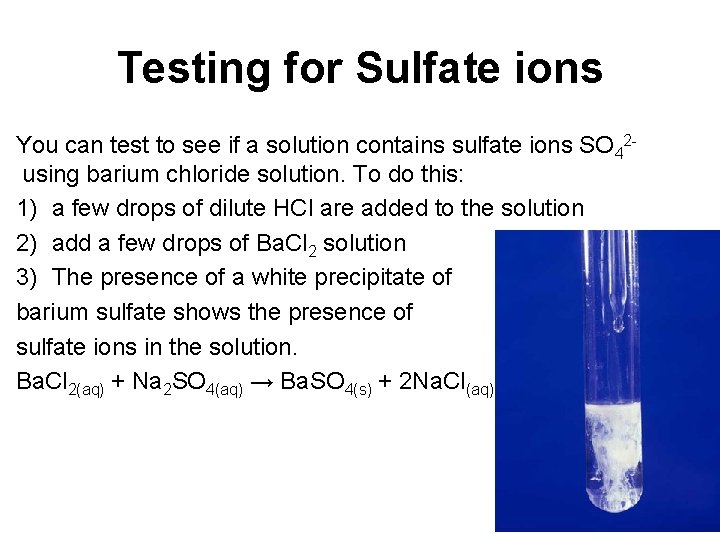
Testing for Sulfate ions You can test to see if a solution contains sulfate ions SO 42 using barium chloride solution. To do this: 1) a few drops of dilute HCl are added to the solution 2) add a few drops of Ba. Cl 2 solution 3) The presence of a white precipitate of barium sulfate shows the presence of sulfate ions in the solution. Ba. Cl 2(aq) + Na 2 SO 4(aq) → Ba. SO 4(s) + 2 Na. Cl(aq)

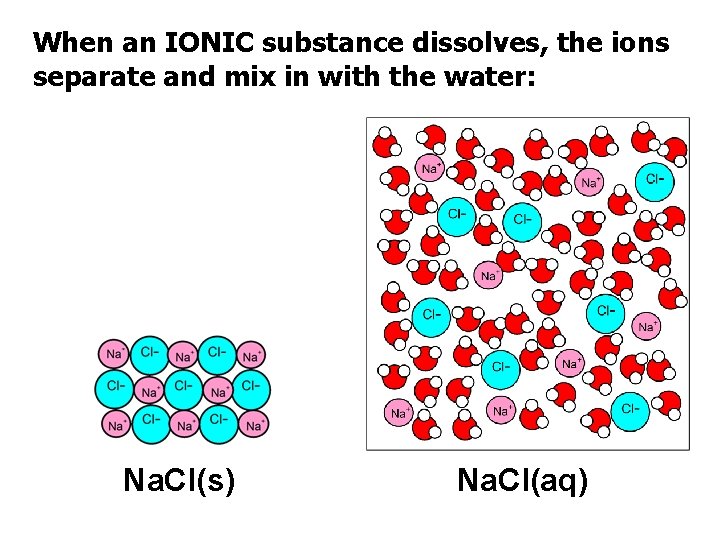
When an IONIC substance dissolves, the ions separate and mix in with the water: Na. Cl(s) Na. Cl(aq)

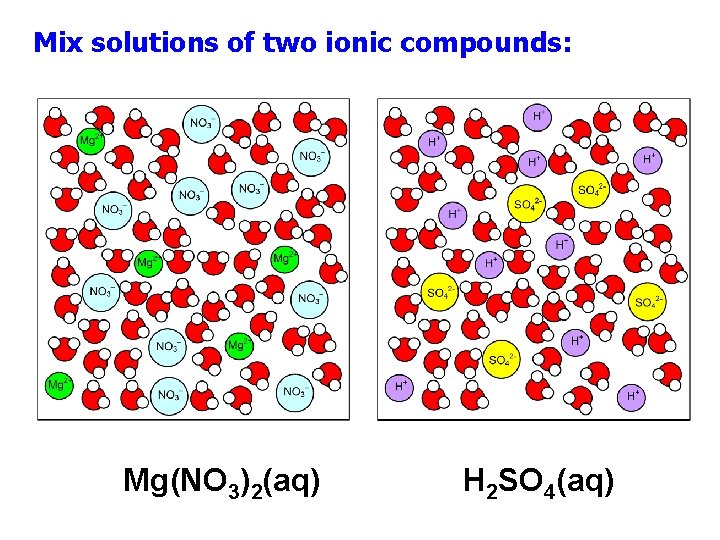
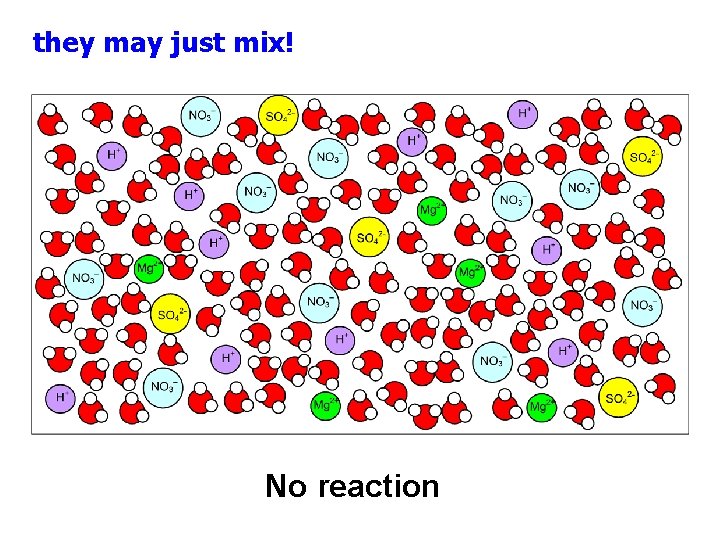
Mix solutions of two ionic compounds: Mg(NO 3)2(aq) H 2 SO 4(aq)

they may just mix! No reaction

Predicting Double Replacement Reactions You could have used your knowledge of solubility to predict whether the previous reaction and if the subsequent reactions are spontaneous.

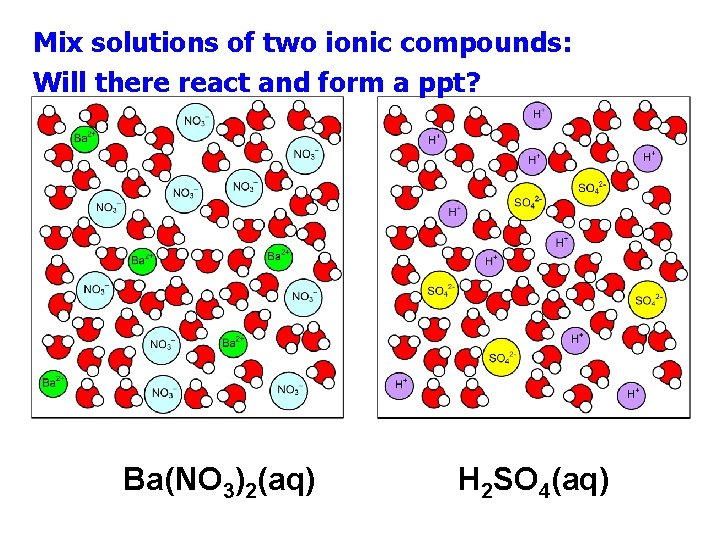
Mix solutions of two ionic compounds: Will there react and form a ppt? Ba(NO 3)2(aq) H 2 SO 4(aq)

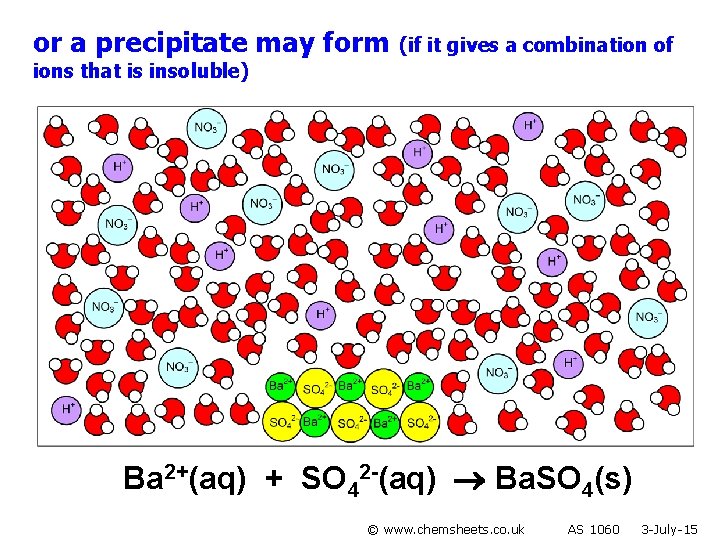
or a precipitate may form (if it gives a combination of ions that is insoluble) Ba 2+(aq) + SO 42 -(aq) Ba. SO 4(s) © www. chemsheets. co. uk AS 1060 3 -July-15

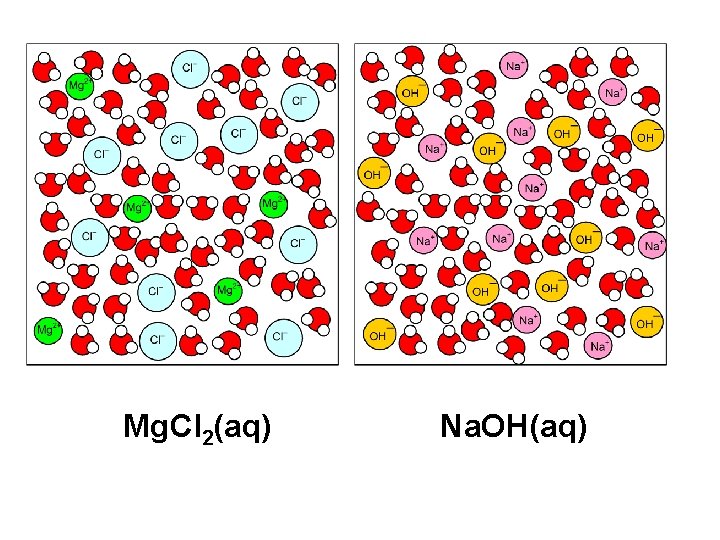
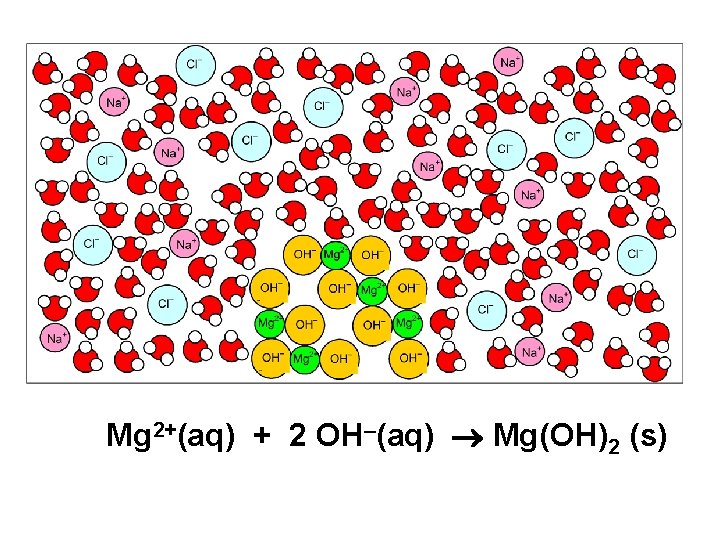
Mg. Cl 2(aq) Na. OH(aq)

Mg 2+(aq) + 2 OH–(aq) Mg(OH)2 (s)

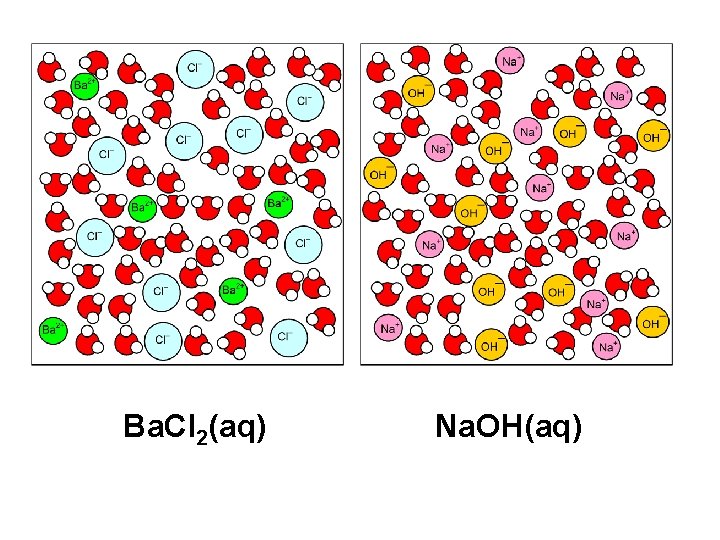
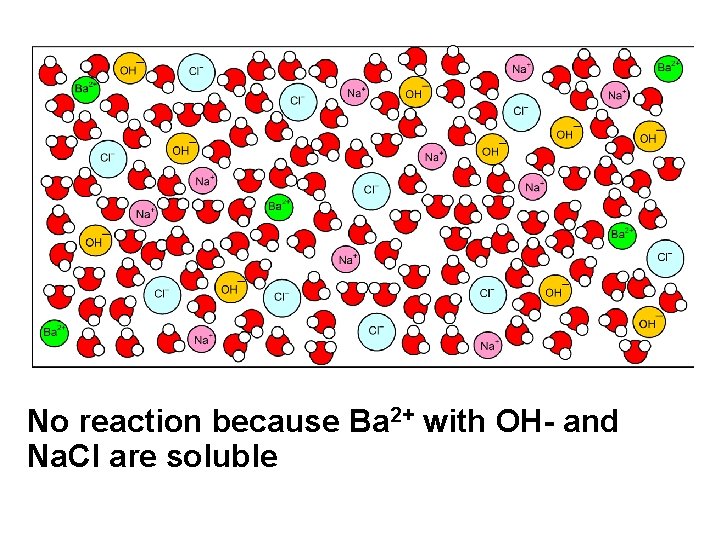
Ba. Cl 2(aq) Na. OH(aq)

No reaction because Ba 2+ with OH- and Na. Cl are soluble

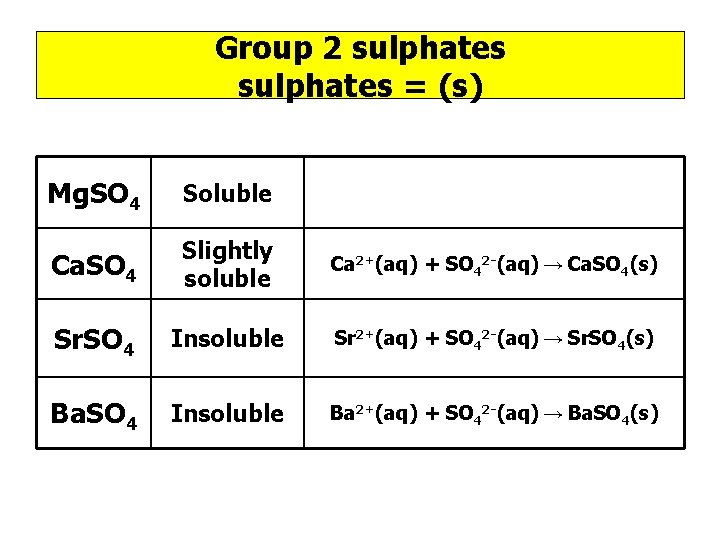
Group 2 sulphates = (s) Mg. SO 4 Soluble Ca. SO 4 Slightly soluble Ca 2+(aq) + SO 42 -(aq) → Ca. SO 4(s) Sr. SO 4 Insoluble Sr 2+(aq) + SO 42 -(aq) → Sr. SO 4(s) Ba. SO 4 Insoluble Ba 2+(aq) + SO 42 -(aq) → Ba. SO 4(s)

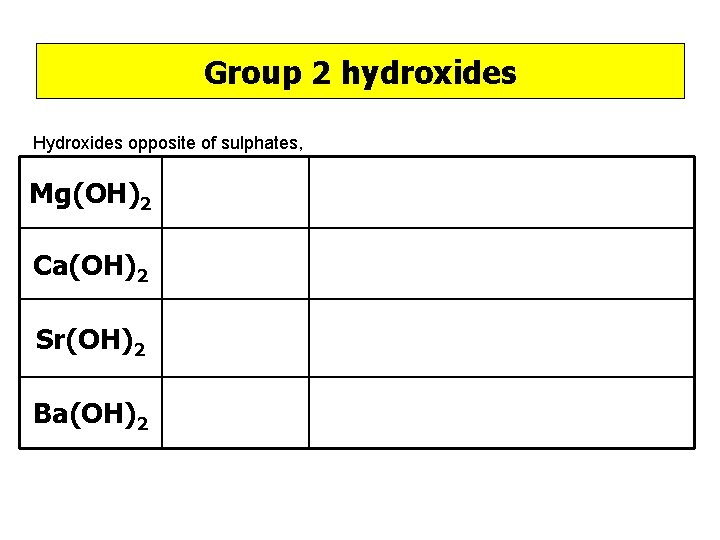
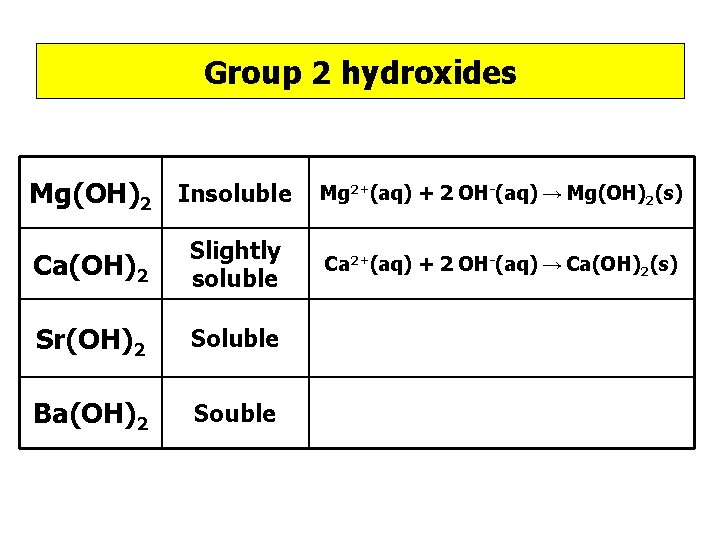
Group 2 hydroxides Hydroxides opposite of sulphates, Mg(OH)2 Ca(OH)2 Sr(OH)2 Ba(OH)2

Group 2 hydroxides Mg(OH)2 Insoluble Ca(OH)2 Slightly soluble Sr(OH)2 Soluble Ba(OH)2 Souble Mg 2+(aq) + 2 OH-(aq) → Mg(OH)2(s) Ca 2+(aq) + 2 OH-(aq) → Ca(OH)2(s)

more soluble Mg. SO 4 Mg(OH)2 Ca. SO 4 Ca(OH)2 Sr. SO 4 Sr(OH)2 Ba. SO 4 Ba(OH)2 Remember - Opposite trends - Ca(OH)2 & Ca. SO 4 sparingly soluble - Ba. SO 4 insoluble more soluble

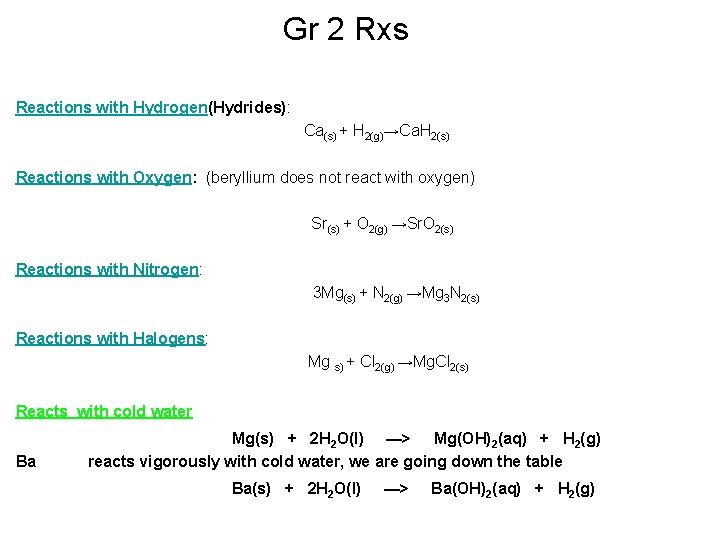
Gr 2 Rxs Reactions with Hydrogen(Hydrides): Ca(s) + H 2(g)→Ca. H 2(s) Reactions with Oxygen: (beryllium does not react with oxygen) Sr(s) + O 2(g) →Sr. O 2(s) Reactions with Nitrogen: 3 Mg(s) + N 2(g) →Mg 3 N 2(s) Reactions with Halogens: Mg s) + Cl 2(g) →Mg. Cl 2(s) Reacts with cold water Ba Mg(s) + 2 H 2 O(l) —> Mg(OH)2(aq) + H 2(g) reacts vigorously with cold water, we are going down the table Ba(s) + 2 H 2 O(l) —> Ba(OH)2(aq) + H 2(g)

CHEMICAL PROPERTIES OF THE ELEMENTS Reactivity increases down the Group due to the ease of cation formation WATER react with increasing vigor down the group Mg reacts very slowly with cold water Mg(s) + 2 H 2 O(l) —> Mg(OH)2(aq) + H 2(g) but reacts quickly with steam (steam is hot) Mg(s) + H 2 O(g) —> Mg. O(s) + H 2(g) Ba reacts vigorously with cold water Ba(s) + 2 H 2 O(l) —> Ba(OH)2(aq) + H 2(g) Next USES…………………. .

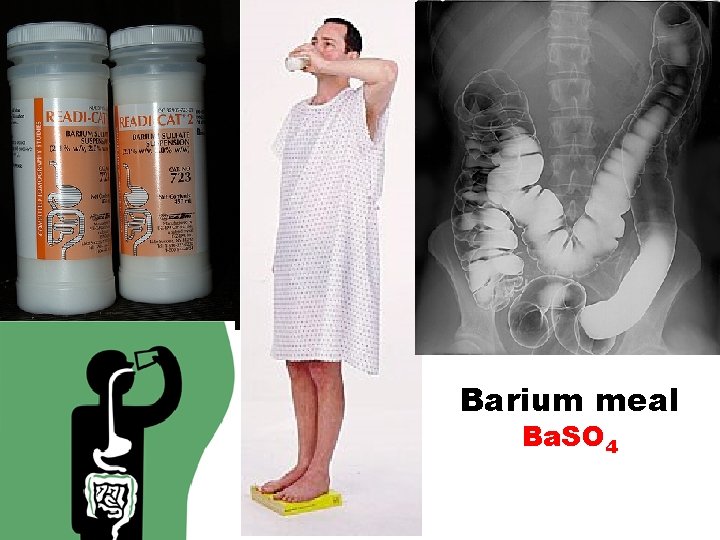
Barium meal Ba. SO 4

Uses of Ca(OH) 2 Calcium hydroxide or slacked lime: 1) Used as limewater to test for CO 2 2) Cement 3) p. H adjustment, 4) digestion aid ………. . as is Mg(OH) 2 See next slides for uses

Slaked lime Ca(OH)2

Limestone / calcium carbonate Ca. CO 3

Milk of magnesia Mg(OH)2