Group 1 The Alkali Metals L O to






















- Slides: 34

Group 1: The Alkali Metals L. O: to know some of the properties of the alkali metals, and to understand their reactivity. 21 November 2020

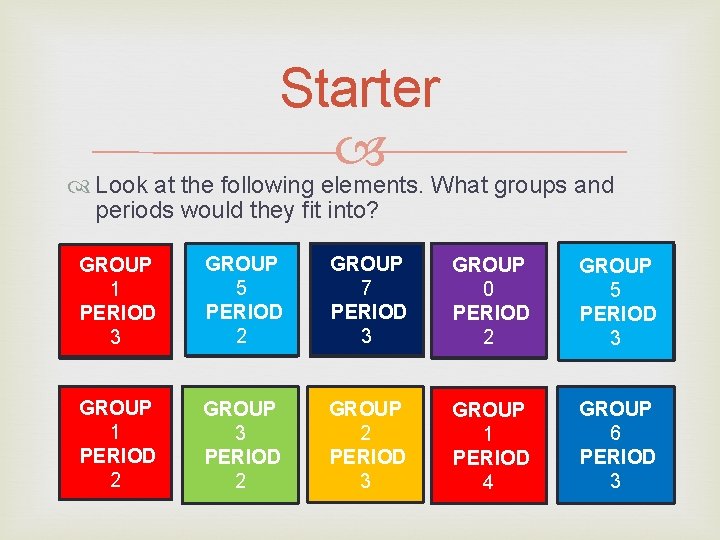
Starter Look at the following elements. What groups and periods would they fit into? GROUP 1 PERIOD 3 GROUP 5 PERIOD 2 GROUP 7 PERIOD 3 GROUP 0 PERIOD 2 GROUP 5 PERIOD 3 GROUP 1 PERIOD 2 GROUP 3 PERIOD 2 GROUP 2 PERIOD 3 GROUP 1 PERIOD 4 GROUP 6 PERIOD 3

Properties The alkali metals are the elements in Group 1 of the periodic table — they are lithium, sodium, potassium, rubidium, caesium and francium. They’re all silvery solids that have to be stored in oil and handled with forceps (they burn the skin). The alkali metals all have one outer electron. This makes them very reactive and gives them all similar properties. For example, the alkali metals all have low density. In fact, the first three in the group are less dense than water.

Trends in Group 1 There a couple of trends within the Group 1 elements that you need to know about. Reactivity increases down the group, so elements at the bottom of the group are more reactive than elements at the top of the group. Q: Why does reactivity increase as you go down the group?

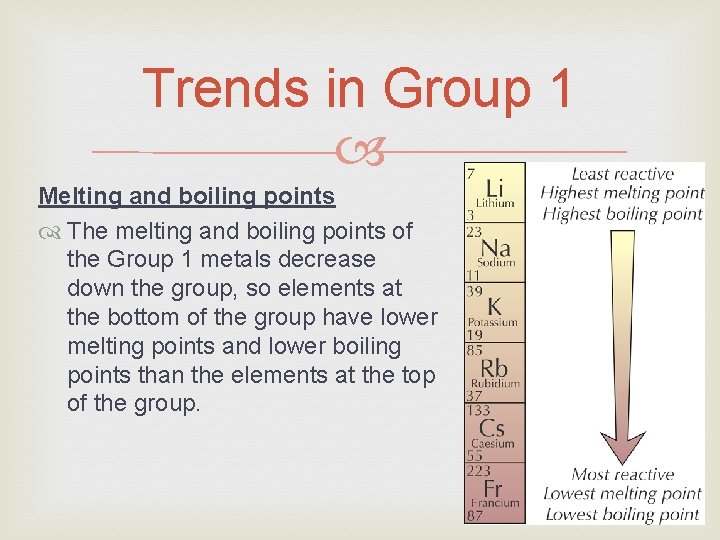
Trends in Group 1 Melting and boiling points The melting and boiling points of the Group 1 metals decrease down the group, so elements at the bottom of the group have lower melting points and lower boiling points than the elements at the top of the group.

Reaction with nonmetals The alkali metals react with non-metals to form ionic compounds.

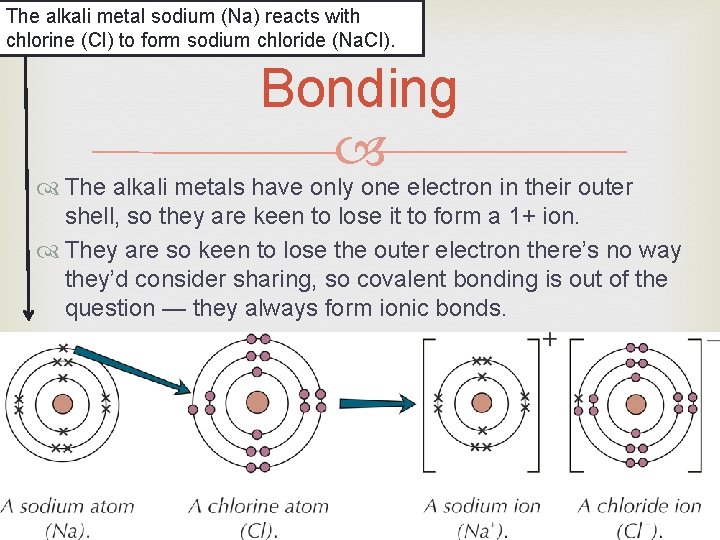
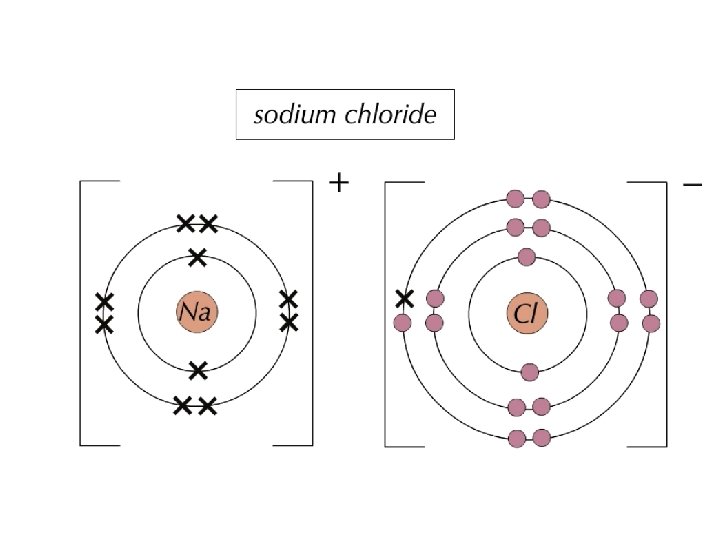
The alkali metal sodium (Na) reacts with chlorine (Cl) to form sodium chloride (Na. Cl). Bonding The alkali metals have only one electron in their outer shell, so they are keen to lose it to form a 1+ ion. They are so keen to lose the outer electron there’s no way they’d consider sharing, so covalent bonding is out of the question — they always form ionic bonds.


Products The compounds that are produced when alkali metals react with non-metals are usually white solids that dissolve in water to form colourless solutions. E. g: Sodium chloride is a white solid that will dissolve in water to form a colourless solution.

Reaction with Water The alkali metals react with water to form a metal hydroxide and hydrogen gas. The general equation for this reaction is: Q 1: What would the products be if I reacted sodium with water? A 1: sodium hydroxide + hydrogen Q 2: What about if I reacted potassium with water? A 2: potassium hydroxide + hydrogen

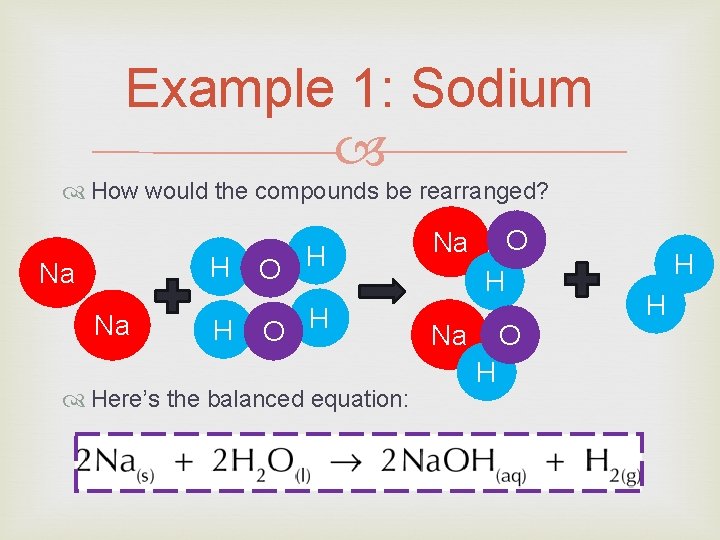
Example 1: Sodium How would the compounds be rearranged? H Na Na H O H Here’s the balanced equation: O Na H Na O H H H

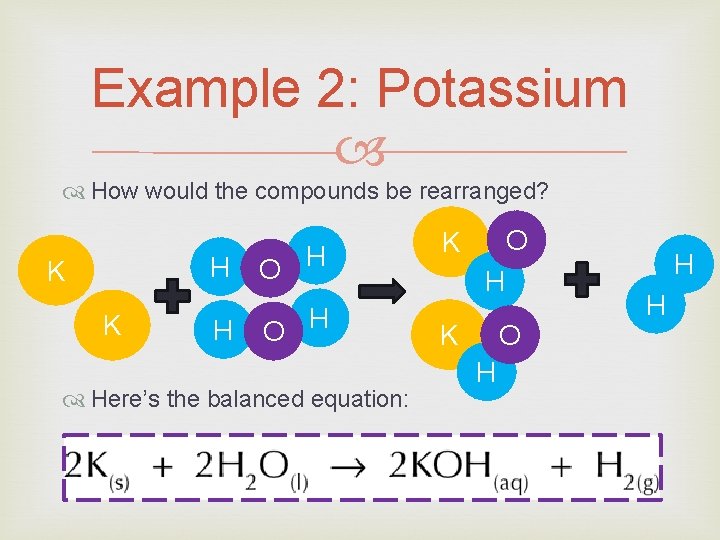
Example 2: Potassium How would the compounds be rearranged? H K K H O H Here’s the balanced equation: O K H K O H H H

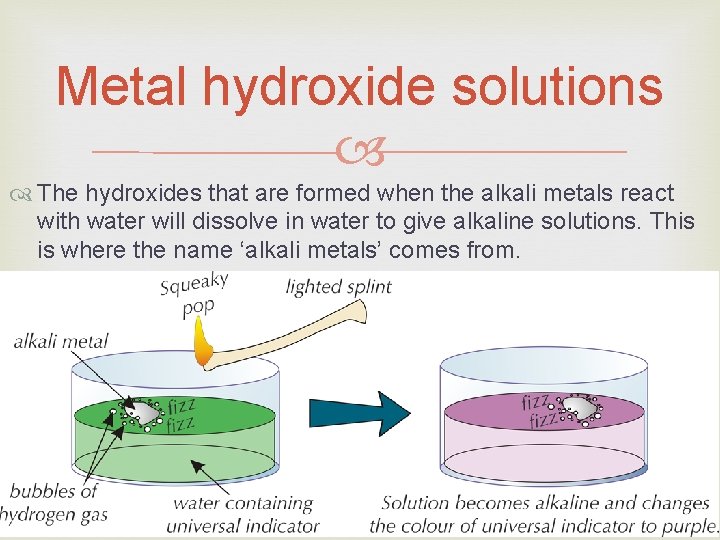
Sodium reacting with water. Reaction with Water Because the alkali metals are so reactive, they react with water very vigorously. When lithium, sodium or potassium are put in water, they float and move around the surface, fizzing furiously as the hydrogen gas is produced. In some cases, the reaction can get hot enough to ignite the hydrogen. Elements below potassium in Group 1 react explosively with water.

Metal hydroxide solutions The hydroxides that are formed when the alkali metals react with water will dissolve in water to give alkaline solutions. This is where the name ‘alkali metals’ comes from.

Flash Quiz Put your hand up to answer the question before the atoms collide!

Q 1: Which group in the periodic table are the alkali metals? Time’s up! RESTAR T ANSWER

Answer: Group 1 NEXT

Q 2: Do the alkali metals have low density or high density? Time’s up! RESTAR T ANSWER

Answer: Low density NEXT

Q 3: State the trend in reactivity as you go down Group 1. Time’s up! RESTAR T ANSWER

Answer: Reactivity increases down group 1 NEXT

Q 4: Which alkali metals have the lowest boiling points — those at the top of Group 1 or those at the bottom of Group 1? Time’s up! RESTAR T ANSWER

Answer: Those at the bottom of group 1 NEXT

Q 5: Alkali metals can react with non-metals. What type of bonds do alkali metals form during these reactions? Time’s up! RESTAR T ANSWER

Answer: ionic bonds NEXT

Q 6: Give two properties of the compounds that are produced during these reactions. Time’s up! RESTAR T ANSWER

Answer: Any two from: e. g. they are white. / They are solids. / They are soluble. / They dissolve in water to give colourless solutions. NEXT

Q 7: What is the general word equation for the reaction of an alkali metal with water? Time’s up! RESTAR T ANSWER

Answer: Alkali metal + water metal hydroxide + hydrogen NEXT

Q 8: Will the solution formed when a Group 1 metal reacts with water be acidic, neutral or alkaline? Time’s up! RESTAR T ANSWER

Answer: Alkaline

Questions 1. Which is more reactive: a) sodium or potassium? b) lithium or rubidium? 2. Which has the higher melting point: a) lithium or potassium? b) caesium or potassium? 3. Is potassium oxide a covalent compound or an ionic compound? 4. Write a balanced symbol equation for the reaction of lithium with water. Include state symbols in your answer.

Answers 1. 2. 3. 4. a) Potassium b) Rubidium a) Lithium b) Potassium An ionic compound. 2 Li(s) + 2 H 2 O(l) 2 Li. OH(aq) + H 2(g)

Homework Complete the table about the elements in group 1. You will need to include: the symbol of each element The electronic structure of each element The properties of each element The uses of each element Some extra information about each element (e. g. who discovered it, when, and some interesting facts about it) Due in: Next lesson