Ground vs Excited State o Ground State electrons




- Slides: 14

Ground vs. Excited State o Ground State – electrons are in positions of lowest energy possible (normal) o Excited State – electron is in a temporary position of higher energy than ground state n Very unstable; the electron quickly returns to the ground state

Compared to a sodium atom in the ground state, a sodium atom in the excited state must have There is not change in the number of electrons, just their location A. ) a greater number of electrons B. ) a smaller number of electrons C. ) an electron with greater energy D. ) an electron with less energy

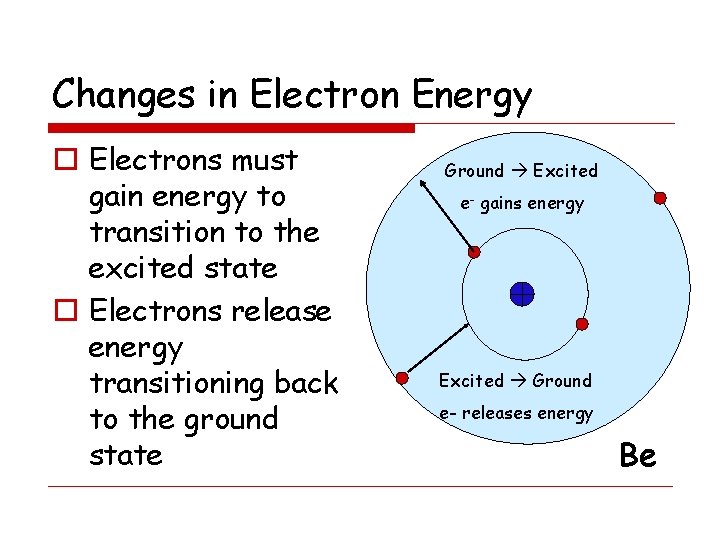
Changes in Electron Energy o Electrons must gain energy to transition to the excited state o Electrons release energy transitioning back to the ground state Ground Excited e- gains energy Excited Ground e- releases energy Be

Which electron transition represents a gain of energy? The farther from the nucleus the greater the energy A. ) from 2 nd to 3 rd shell B. ) from 2 nd to 1 st shell C. ) from 3 rd to 2 nd shell D. ) from 3 rd to 1 st shell

Emission of Energy o All elements have a unique energy level system (due to differences in nuclear charge) o The amount of energy released when returning from excited state to ground state is different for each element n Multiple transitions are possible o Some of the energy released has wavelengths within the visible light range

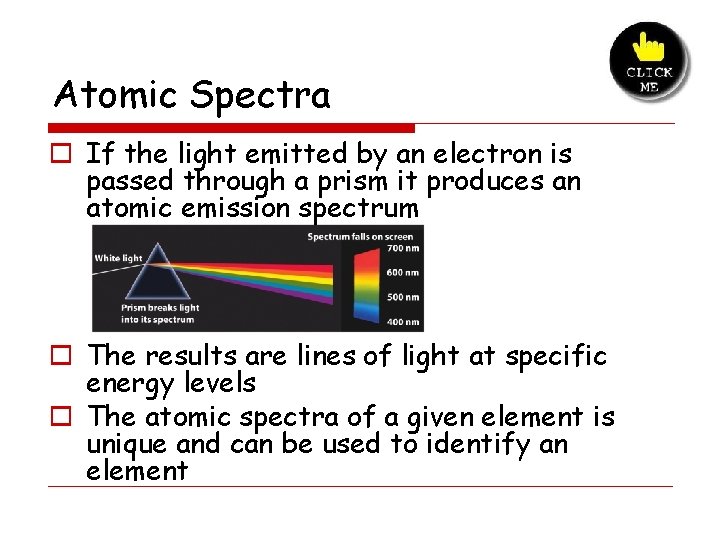
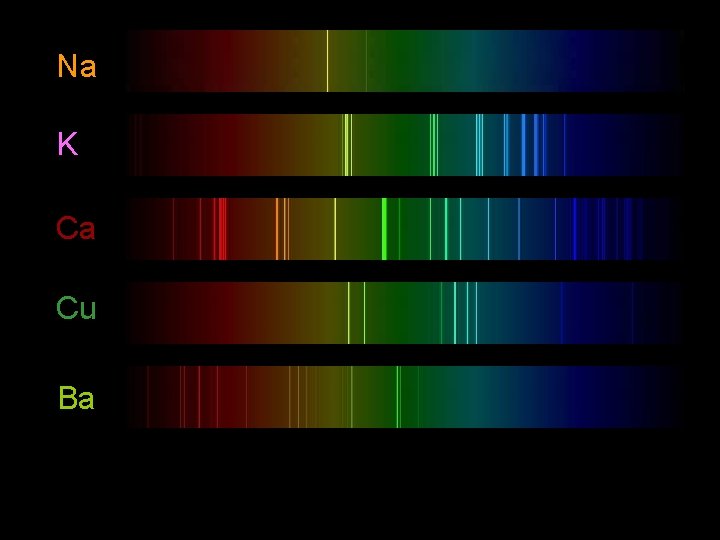
Atomic Spectra o If the light emitted by an electron is passed through a prism it produces an atomic emission spectrum o The results are lines of light at specific energy levels o The atomic spectra of a given element is unique and can be used to identify an element

Na K Ca Cu Ba

The characteristic bright-line spectrum of an element occurs when electrons A. ) move from lower to higher energy levels B. ) move from higher to lower energy levels C. ) are lost by a neutral atom D. ) are gained by a neutral atom High to low releases energy

The characteristic spectral lines of elements are caused when electrons in an excited atom move from A. ) lower to higher energy levels, releasing energy B. ) lower to higher energy levels, absorbing energy C. ) higher to lower energy levels, releasing energy D. ) higher to lower energy levels, absorbing energy

Electron X can change to a higher energy level or a lower energy level. Which statement is true of electron X? A. ) Electron X emits energy when it changes to a higher energy level. B. ) Electron X absorbs energy when it changes to a higher energy level. C. ) Electron X absorbs energy when it changes to a lower energy level. D. ) Electron X neither emits nor absorbs energy when it changes energy level.

Which of the following quantum leaps would be associated with the greatest energy of emitted light? A. ) n = 5 to n = 1 B. ) n = 4 to n = 5 C. ) n = 2 to n = 5 D. ) n = 5 to n = 4

The atomic emission spectra of a sodium atom on Earth and of a sodium atom in the sun would be A. ) the same B. ) different from each other C. ) the same as those of several other elements D. ) the same as each other only in the ultraviolet range Each element has a unique spectra that is unchanged based on locations

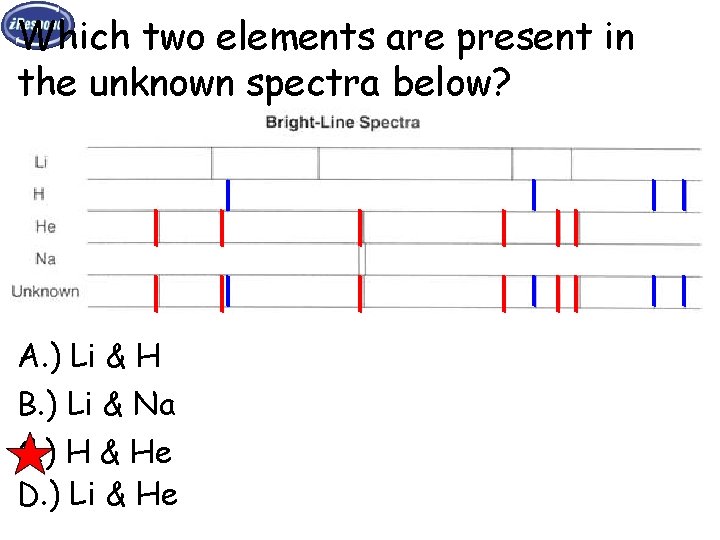
Which two elements are present in the unknown spectra below? A. ) Li & H B. ) Li & Na C. ) H & He D. ) Li & He

Electron Cumulative Quiz o o Valence Electrons Lewis Dot Diagrams Ground Vs. Excited State Bright Line Spectra