Green Chemistry Chapter 5 DEFINITION OF GREEN OR















![Biomaterials [Carbohydrates, Proteins, Lipids] Highly Functionalized Molecules Petroleum Products [Hydrocarbons] Singly Functionalized Compounds [Olefins, Biomaterials [Carbohydrates, Proteins, Lipids] Highly Functionalized Molecules Petroleum Products [Hydrocarbons] Singly Functionalized Compounds [Olefins,](https://slidetodoc.com/presentation_image_h/ee509e705daa2d5cc196d66d590fd790/image-23.jpg)









- Slides: 35

Green Chemistry Chapter 5

DEFINITION OF GREEN OR SUSTAINABLE CHEMISTRY • An approach to design, manufacture and use of chemical products to deliberately reduce or eliminate the chemical hazards and protect the environment.

Basic Principles of Green Chemistry • 12 principles advocated by Dr. Paul Anastas and Dr. John Warner. 1. Prevention 2. Atomic Economy 3. Less Hazardous Chemical Synthesis 4. Designing Safer Chemicals 5. Safer Solvents and Auxiliaries 6. Design for energy efficiency

7. Use of Renewable Feedstocks 8. Reduce Derivatives 9. Catalysis 10. Design for Degradation 11. Real –Time analysis for Pollution prevention 12. Essentially Safer Chemistry for Accident Prevention

1. Prevention • It is better to prevent waste than to treat or clean up waste after created it. • So, waste prevention is better than waste cleanup.

Environmental Disasters • Love Canal In Niagara Falls, NY a chemical and plastics company had used an old canal bed as a chemical dump from 1930 s to 1950 s. The land was then used for a new school and housing track. The chemicals leaked through a clay cap that sealed the dump. It was contaminated with at least 82 chemicals (benzene, chlorinated hydrocarbons, dioxin). Health effects of the people living there included: high birth defect incidence and seizure-inducing nervous disease among the children.

• Cuyahoga River – Cleveland, Ohio – There were many things being dumped in the river such as: gasoline, oil, paint, and metals. The river was called "a rainbow of many different colors". Some river! Chocolate-brown, oily, bubbling with subsurface gases, it oozes rather than flows. "Anyone who falls into the Cuyahoga does not drown, " Cleveland's citizens joke grimly. "He decays. " Time Magazine, August 1969

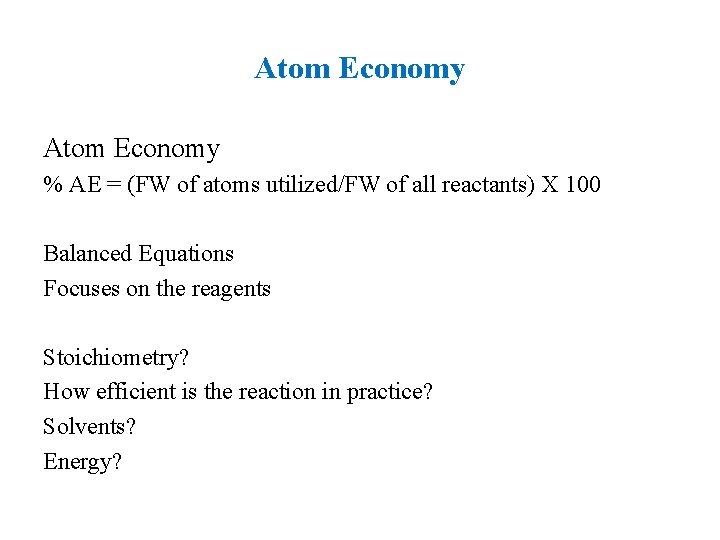
2. Atomic Economy Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.

Atom Economy % AE = (FW of atoms utilized/FW of all reactants) X 100 Balanced Equations Focuses on the reagents Stoichiometry? How efficient is the reaction in practice? Solvents? Energy?

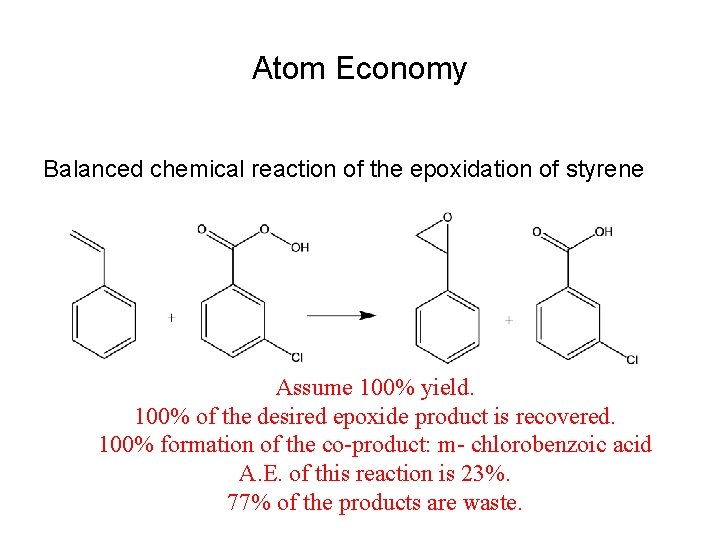
Atom Economy Balanced chemical reaction of the epoxidation of styrene Assume 100% yield. 100% of the desired epoxide product is recovered. 100% formation of the co-product: m- chlorobenzoic acid A. E. of this reaction is 23%. 77% of the products are waste.

3. Less Hazardous Chemical Synthesis • Synthetic routes should be designed in such a way that, it generates and uses substances that possess no or little toxicity to human health and the environment.

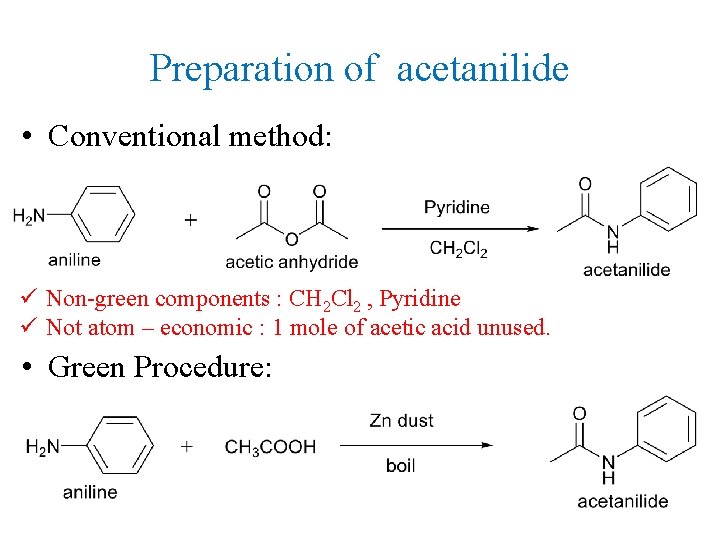
Preparation of acetanilide • Conventional method: ü Non-green components : CH 2 Cl 2 , Pyridine ü Not atom – economic : 1 mole of acetic acid unused. • Green Procedure:

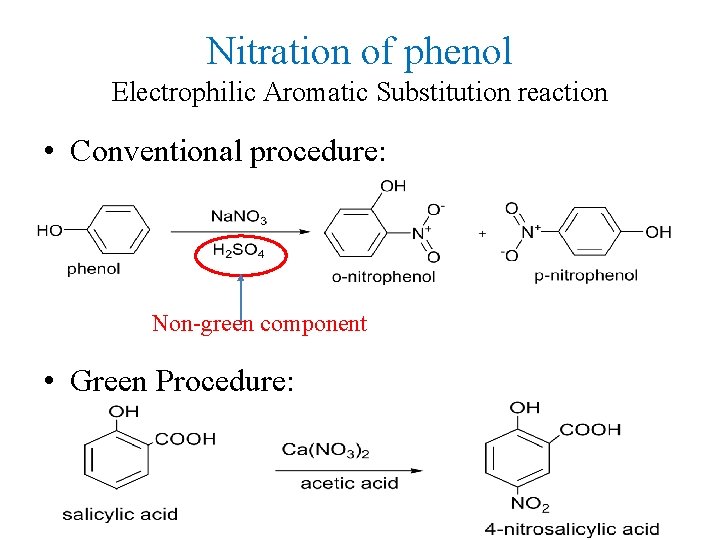
Nitration of phenol Electrophilic Aromatic Substitution reaction • Conventional procedure: Non-green component • Green Procedure:

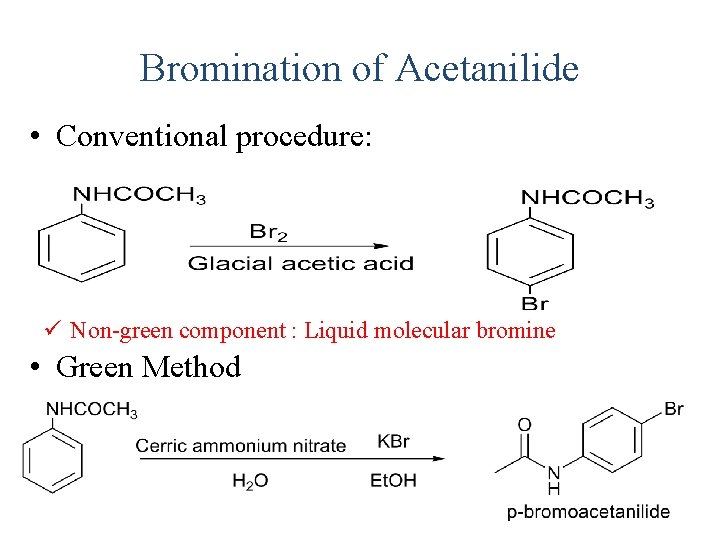
Bromination of Acetanilide • Conventional procedure: ü Non-green component : Liquid molecular bromine • Green Method

4. Designing Safer Chemicals • New chemical should be design with great effectiveness and new approaches to minimize the toxicity and harmless to the environment.

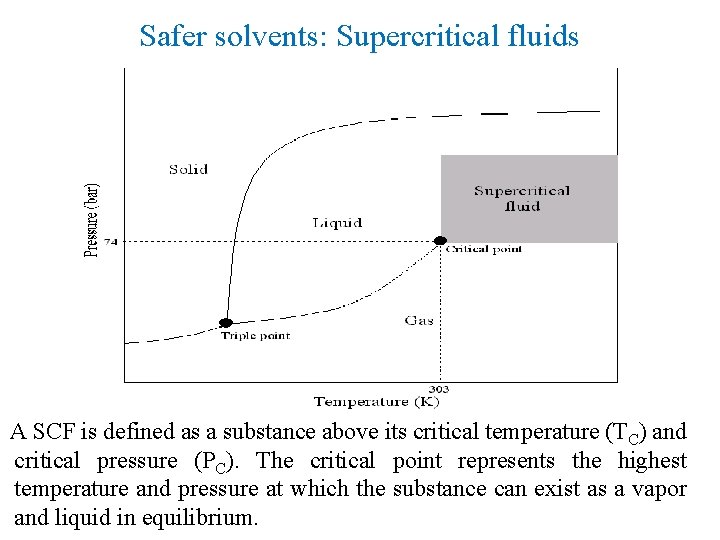
5. Safer Solvents and Auxiliaries • The auxiliary substances (Inorganic and organic solvents) should be replaced by safer and ecofriendly green solvents like IL, supercritical CO 2 fluid, and Supercritical water. • Promote solvent free systems i. e adsorbents like clays, zeolites, silica, and alumina.

Safer solvents: Supercritical fluids A SCF is defined as a substance above its critical temperature (TC) and critical pressure (PC). The critical point represents the highest temperature and pressure at which the substance can exist as a vapor and liquid in equilibrium.

Ionic Liquids (Ils) • Liquid at room temperature and below. • Non-volatile • Negligible vapour pressure • Can be recycled • High thermal stability to 200 o. C or higher • First Room temperature ionic liquid (RTIL) : Ethylammonium Nitrate [Et. NH 3 ]+[NO 3]- was synthesized by Paul Walden (1914) using neutralization method.

Supercritical CO 2 fluid • Have low viscosity • No surface tension • Low toxicity • Non-flammability • Easily evaporated leaving no residue • Can dissolve wide range of chemicals • Used in fragrance compounds

Supercritical water • Water become supercritical at 3740 C and 218 atm. • Use as a green solvent for many synthetic reactions.

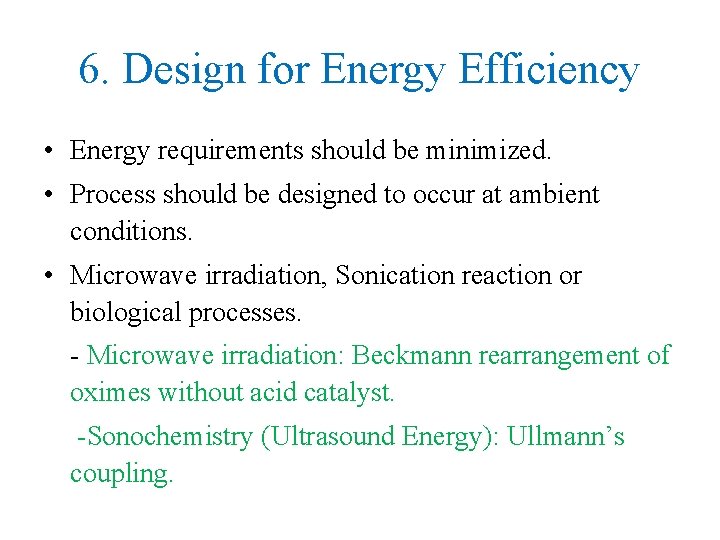
6. Design for Energy Efficiency • Energy requirements should be minimized. • Process should be designed to occur at ambient conditions. • Microwave irradiation, Sonication reaction or biological processes. - Microwave irradiation: Beckmann rearrangement of oximes without acid catalyst. -Sonochemistry (Ultrasound Energy): Ullmann’s coupling.

7. Use of Renewable Feedstocks A raw material or feedstock should be renewable rather than depleting whenever technically and economically practical.
![Biomaterials Carbohydrates Proteins Lipids Highly Functionalized Molecules Petroleum Products Hydrocarbons Singly Functionalized Compounds Olefins Biomaterials [Carbohydrates, Proteins, Lipids] Highly Functionalized Molecules Petroleum Products [Hydrocarbons] Singly Functionalized Compounds [Olefins,](https://slidetodoc.com/presentation_image_h/ee509e705daa2d5cc196d66d590fd790/image-23.jpg)
Biomaterials [Carbohydrates, Proteins, Lipids] Highly Functionalized Molecules Petroleum Products [Hydrocarbons] Singly Functionalized Compounds [Olefins, Alkylchlorides] Highly Functionalized Molecules

8. Reduce Derivatives Unnecessary derivatization (blocking group, protection/deprotection, temporary modification of physical/chemical processes) should be avoided whenever possible.

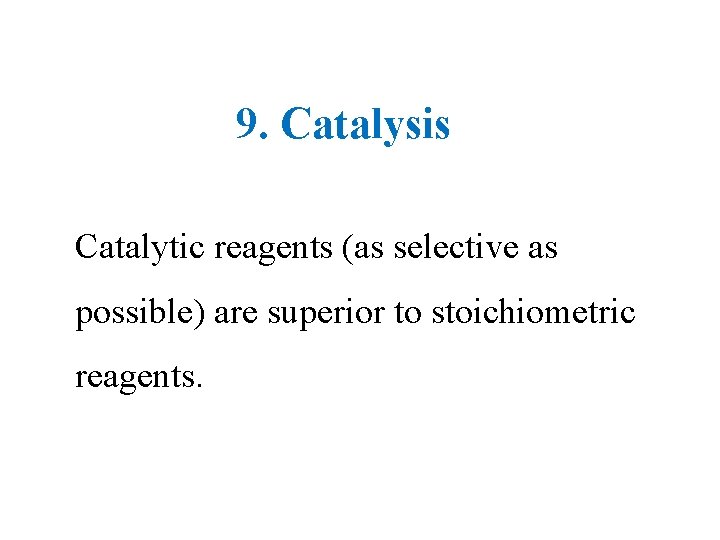
9. Catalysis Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.

10. Design for Degradation Chemical products should be designed so that at the end of their function they do not persist in the environment and instead break down into innocuous degradation products.

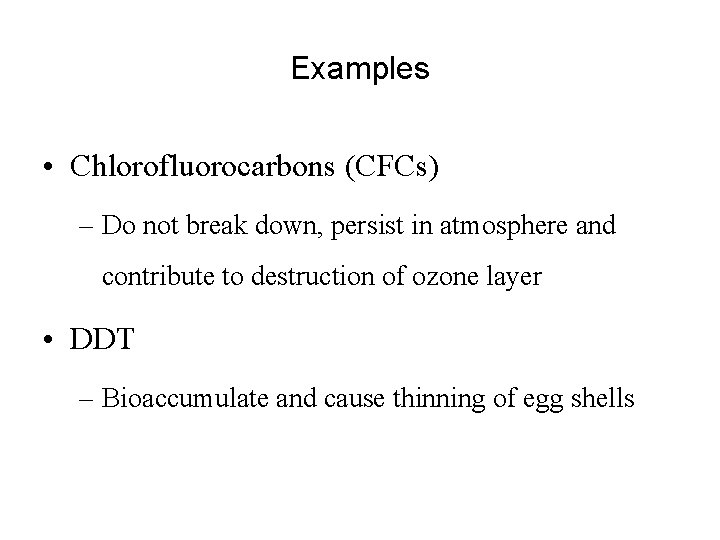
Examples • Chlorofluorocarbons (CFCs) – Do not break down, persist in atmosphere and contribute to destruction of ozone layer • DDT – Bioaccumulate and cause thinning of egg shells

11. Real-time Analysis for Pollution Prevention Analytical methodologies need to be further developed to allow for real-time in-process monitoring and control prior to the formation of hazardous substances.

Real time analysis for a chemist is the process of “checking the progress of chemical reactions as it happens. ” Knowing when your product is “done” can save a lot of waste, time and energy!

Analyzing a Reaction What do you need to know, how do you get this information and how long does it take to get it?

12. Inherently Safer Chemistry for Accident Prevention Substance and the form of a substance used in a chemical process should be chosen so as to minimize the potential for chemical accidents, including releases, explosions, and fires.

Cyanide! Phosgene!

Tragedy in Bhopal, India - 1984 What happened? • Methyl isocyanate – used to make pesticides was being stored in large quantities on-site at the plant • Methyl isocyanate is highly reactive, exothermic molecule • Most safety systems either failed or were inoperative • Water was released into the tank holding the methyl isocyanate • The reaction occurred and the methyl isocyanate rapidly boiled producing large quantities of toxic gas.

Conclusion Not a solution to all environmental problems But Green chemistry the most fundamental approach to preventing pollution.

Thank you
 Show me green
Show me green Define green chemistry
Define green chemistry Green chemistry definition
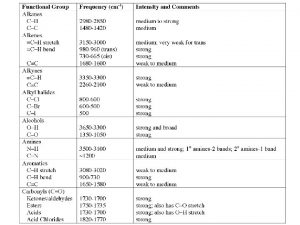
Green chemistry definition Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Atom economy in green chemistry
Atom economy in green chemistry 12 principles of green chemistry with examples
12 principles of green chemistry with examples Antifoulant green chemistry
Antifoulant green chemistry E factor green chemistry
E factor green chemistry Asc green chemistry institute
Asc green chemistry institute Green chemistry book by chandrakanta bandyopadhyay
Green chemistry book by chandrakanta bandyopadhyay Ionic liquids green chemistry ppt
Ionic liquids green chemistry ppt Green chemistry
Green chemistry Conclusion of green chemistry
Conclusion of green chemistry Acs green chemistry
Acs green chemistry Peter dunn pfizer
Peter dunn pfizer Lernpyramide von green & green (2005)
Lernpyramide von green & green (2005) Green yellow blue
Green yellow blue Frc driver station mac
Frc driver station mac Is chalk natural or manmade
Is chalk natural or manmade Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Pericyclic
Pericyclic What is organic chemistry
What is organic chemistry Modern chemistry textbook answers chapter 9
Modern chemistry textbook answers chapter 9 Formula of love
Formula of love Modern chemistry chapter 15
Modern chemistry chapter 15 Modern chemistry chapter 14 review answers
Modern chemistry chapter 14 review answers Chapter 13 ions in aqueous solutions
Chapter 13 ions in aqueous solutions Chapter 12 solutions section 1
Chapter 12 solutions section 1 Chapter 11 review gases section 1
Chapter 11 review gases section 1 Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas What functional group is ch3
What functional group is ch3