GRAVIMETRIC METHODS OF ANALYSIS Gravimetric methods are quantitative













- Slides: 29

GRAVIMETRIC METHODS OF ANALYSIS Gravimetric methods are quantitative methods based upon measuring the mass of a pure compound to which the analyte is chemically related. 1. Precipitation Method • Based on determination of an analyte which is precipitated by a precipitating reagent. • The precipitate is a slightly soluble (low solubility) substance with a known composition or it can be converted to one of known composition. Example: Determination of Cl by the addition of Ag+ to form solid Ag. Cl; Ag+ + Cl Ag. Cl(S) Determination of Ni 2+ by the addition of DMG to form solid Ni(DMG)2 Ni 2+ + DMG Ni(DMG)2

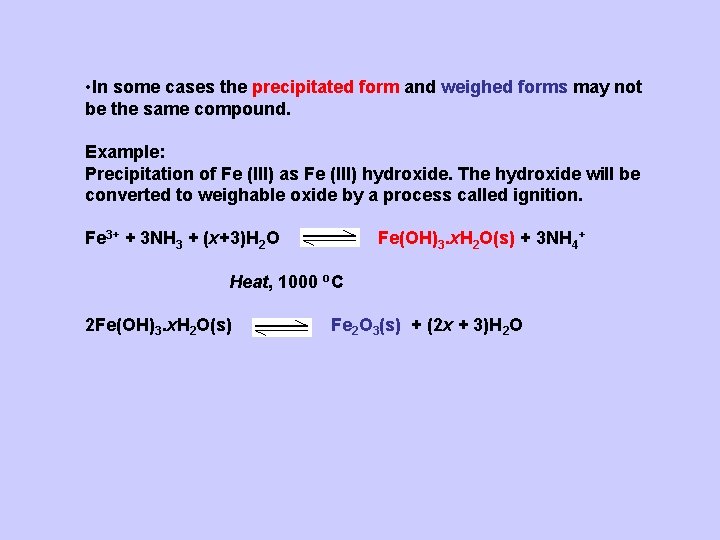
• In some cases the precipitated form and weighed forms may not be the same compound. Example: Precipitation of Fe (III) as Fe (III) hydroxide. The hydroxide will be converted to weighable oxide by a process called ignition. Fe 3+ + 3 NH 3 + (x+3)H 2 O Fe(OH)3. x. H 2 O(s) + 3 NH 4+ Heat, 1000 o. C 2 Fe(OH)3. x. H 2 O(s) Fe 2 O 3(s) + (2 x + 3)H 2 O

2. Volatilization Method • For determination of volatile components (H 2 O, CO 2, etc. ) of a sample. • The sample is warmed or ignited and the amount of the component is found from the loss in mass of the sample. • Or, volatile components can be absorbed by a suitable absorbent. The amount of the components are found from the increase in the weight of the absorbent.

REQUIREMENTS FOR PRECIPITATES The following properties of the precipitate are required in gravimetric methods; • Low solubility, so that the amount of the analyte lost during filtration and washing are negligible (the amount losses is less than 0. 0002 g). • Easily filtered and easily washed free of impurities (the precipitate must be pure). • Unreactive to the atmosphere. • Known composition after drying or ignition. Possible questions: Why we want the precipitates is unreactive to atmosphere? Why we want the precipitates is known composition after drying and ignition?

Factors that influenced the properties of precipitate: p. H Temperature Solubility of the precipitate Precipitating agents What’s the questions we can bring out from this notes:

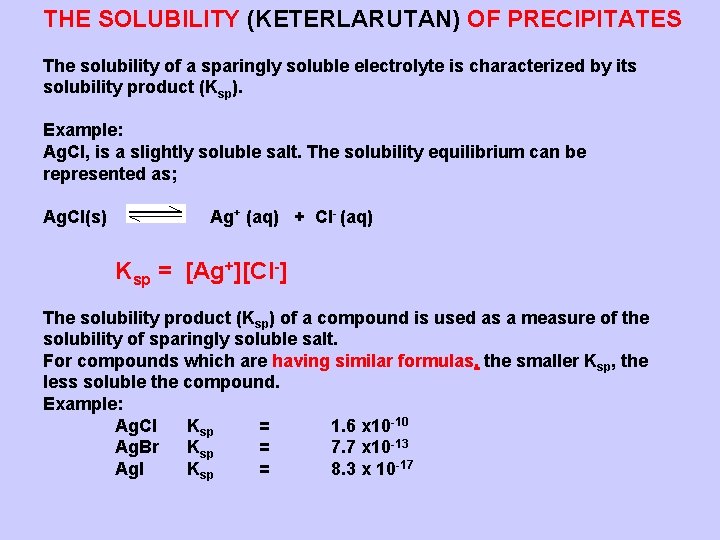
THE SOLUBILITY (KETERLARUTAN) OF PRECIPITATES The solubility of a sparingly soluble electrolyte is characterized by its solubility product (Ksp). Example: Ag. Cl, is a slightly soluble salt. The solubility equilibrium can be represented as; Ag. Cl(s) Ag+ (aq) + Cl- (aq) Ksp = [Ag+][Cl-] The solubility product (Ksp) of a compound is used as a measure of the solubility of sparingly soluble salt. For compounds which are having similar formulas, the smaller Ksp, the less soluble the compound. Example: Ag. Cl Ksp = 1. 6 x 10 -10 Ag. Br Ksp = 7. 7 x 10 -13 Ag. I Ksp = 8. 3 x 10 -17

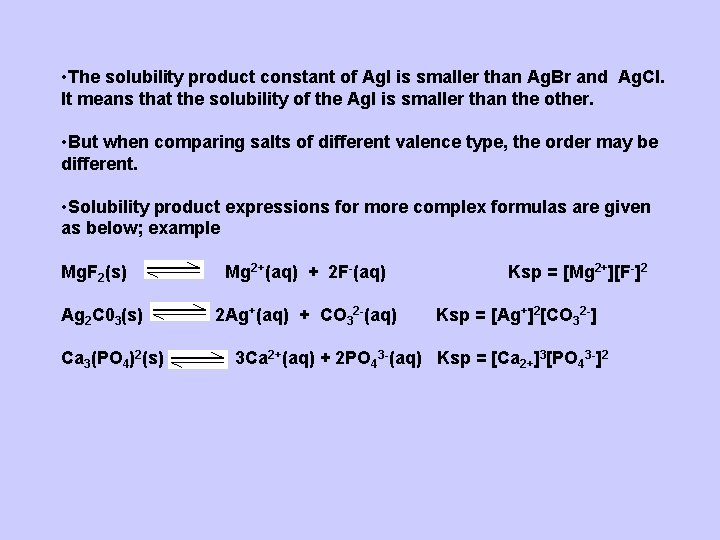
• The solubility product constant of Ag. I is smaller than Ag. Br and Ag. Cl. It means that the solubility of the Ag. I is smaller than the other. • But when comparing salts of different valence type, the order may be different. • Solubility product expressions for more complex formulas are given as below; example Mg. F 2(s) Ag 2 C 03(s) Ca 3(PO 4)2(s) Mg 2+(aq) + 2 F-(aq) 2 Ag+(aq) + CO 32 -(aq) Ksp = [Mg 2+][F-]2 Ksp = [Ag+]2[CO 32 -] 3 Ca 2+(aq) + 2 PO 43 -(aq) Ksp = [Ca 2+]3[PO 43 -]2

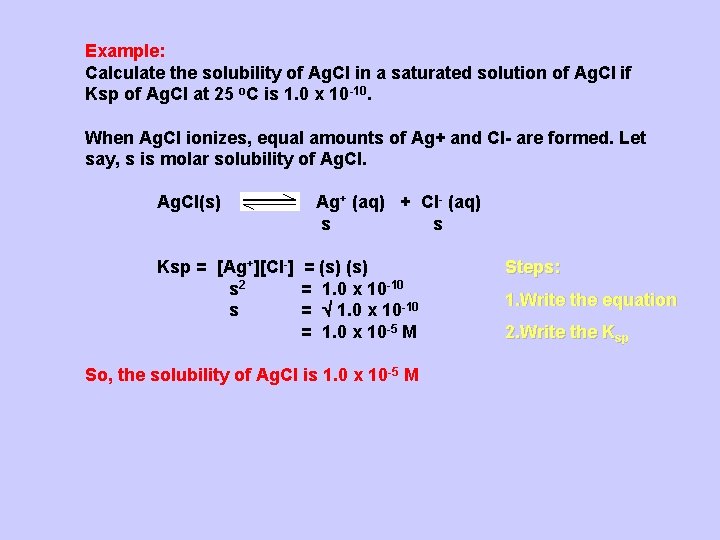
Example: Calculate the solubility of Ag. Cl in a saturated solution of Ag. Cl if Ksp of Ag. Cl at 25 o. C is 1. 0 x 10 -10. When Ag. Cl ionizes, equal amounts of Ag+ and Cl- are formed. Let say, s is molar solubility of Ag. Cl(s) Ag+ (aq) + Cl- (aq) s s Ksp = [Ag+][Cl-] = (s) s 2 = 1. 0 x 10 -10 s = 1. 0 x 10 -10 = 1. 0 x 10 -5 M So, the solubility of Ag. Cl is 1. 0 x 10 -5 M Steps: 1. Write the equation 2. Write the Ksp

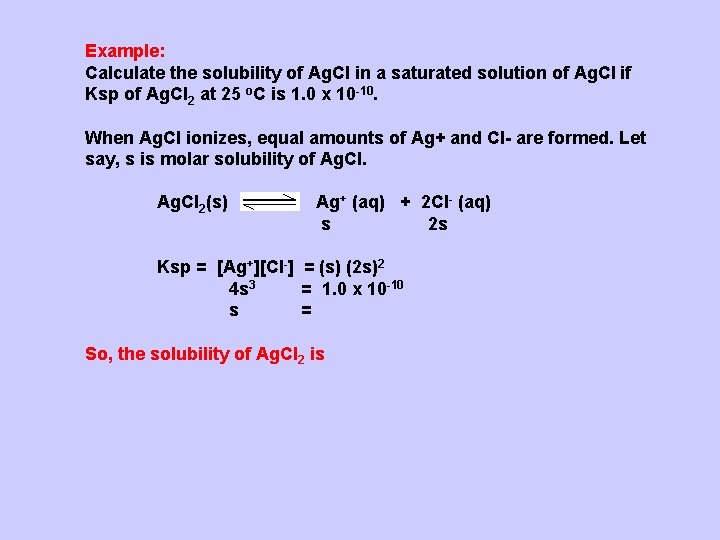
Example: Calculate the solubility of Ag. Cl in a saturated solution of Ag. Cl if Ksp of Ag. Cl 2 at 25 o. C is 1. 0 x 10 -10. When Ag. Cl ionizes, equal amounts of Ag+ and Cl- are formed. Let say, s is molar solubility of Ag. Cl 2(s) Ag+ (aq) + 2 Cl- (aq) s 2 s Ksp = [Ag+][Cl-] = (s) (2 s)2 4 s 3 = 1. 0 x 10 -10 s = So, the solubility of Ag. Cl 2 is

Example: Which has greater solubility in water: Ag. IO 3 (Ksp = 3. 0 x 10 -8) or La(IO 3)3 (Ksp= 6. 5 x 10 -12) ? Ag. IO 3 Ag+ + IO 3 s s Ksp = [Ag+][ IO 3 -] = 3. 0 x 10 -8 [Ag+] = [ IO 3 -] = s (s) = 3. 0 x 10 -8 s = 3. 0 x 10 -8 = 1. 73 x 10 -4 mol L-1 La(IO 3)3 La 3+ + 3 IO 3 s 3 s [La 3+] [IO 3 - ]3 = 6. 5 x 10 -12 (s) (3 s)3 = 6. 5 x 10 -12 27 s 4 = 6. 5 x 10 -12 s = 4 6. 5 x 10 -12 / 27 = 7. 0 x 10 -4 mol L-1 Although the Ksp for Ag. IO 3 is greater than that for La(IO 3)3, but La(IO 3)3 is more soluble.

EFFECT OF TEMPERATURE ON COMPLETENESS OF PRECIPITATION • The solubility product depends on temperature. If the temperature alters, the solubility product of the precipitate also changes. • For example, the solubility of Ag. Cl at 100 o. C is nearly 25 times as high as at 10 o. C. • But some precipitate like Ba. SO 4, the solubility only doubled when the temperature is raised from 10 o. C to 100 o. C. • In some instances the solubility of precipitate decreases with rise of temperature.

EFFECT OF PH ON COMPLETENESS OF PRECIPITATION • p. H of solution influences the degree of precipitation. Example: Mg(OH)2(s) Mg 2+(aq) + 2 OH-(aq) Adding OH- ions (increasing the p. H) shifts the equilibrium from right to left, thereby increasing the precipitation (decreasing the solubility) of Mg(OH)2. Adding H+ ions (decreasing the p. H) shifts the equilibrium from left to right and the solubility of Mg(OH)2 increases.

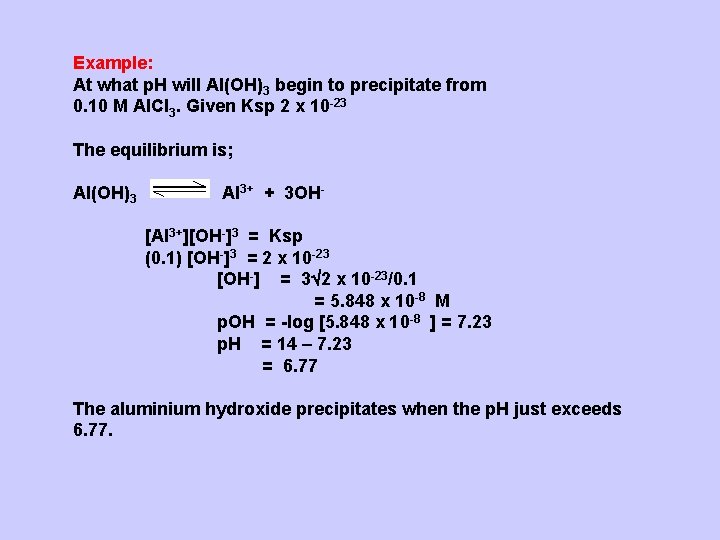
Example: At what p. H will Al(OH)3 begin to precipitate from 0. 10 M Al. Cl 3. Given Ksp 2 x 10 -23 The equilibrium is; Al(OH)3 Al 3+ + 3 OH[Al 3+][OH-]3 = Ksp (0. 1) [OH-]3 = 2 x 10 -23 [OH-] = 3 2 x 10 -23/0. 1 = 5. 848 x 10 -8 M p. OH = -log [5. 848 x 10 -8 ] = 7. 23 p. H = 14 – 7. 23 = 6. 77 The aluminium hydroxide precipitates when the p. H just exceeds 6. 77.

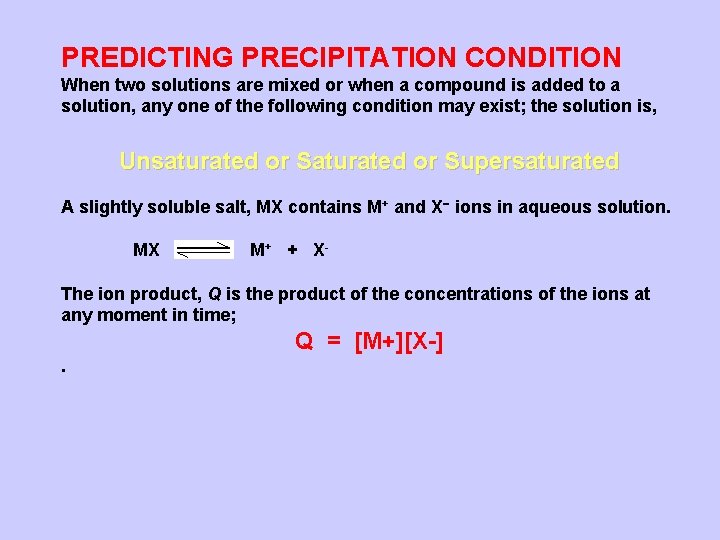
PREDICTING PRECIPITATION CONDITION When two solutions are mixed or when a compound is added to a solution, any one of the following condition may exist; the solution is, Unsaturated or Supersaturated A slightly soluble salt, MX contains M+ and X ions in aqueous solution. MX M+ + X- The ion product, Q is the product of the concentrations of the ions at any moment in time; Q = [M+][X-].

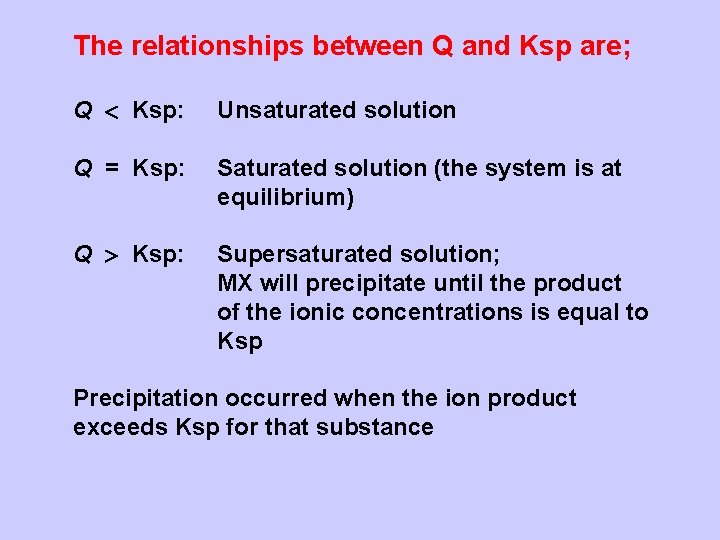
The relationships between Q and Ksp are; Q Ksp: Unsaturated solution Q = Ksp: Saturated solution (the system is at equilibrium) Q Ksp: Supersaturated solution; MX will precipitate until the product of the ionic concentrations is equal to Ksp Precipitation occurred when the ion product exceeds Ksp for that substance

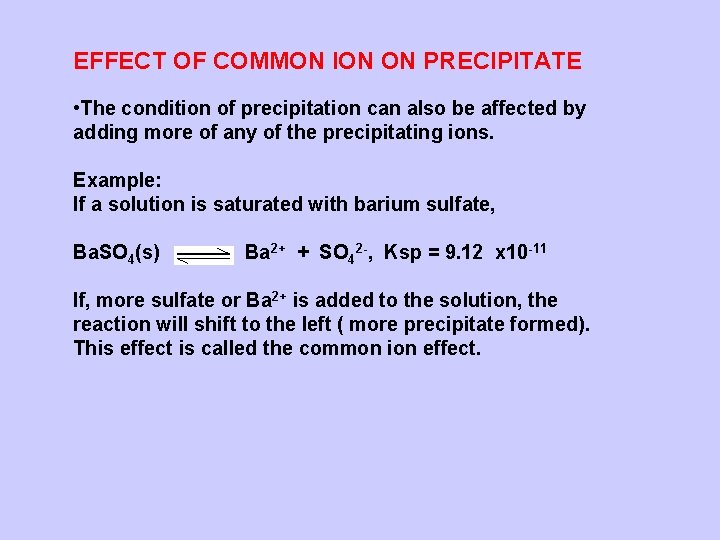
EFFECT OF COMMON ION ON PRECIPITATE • The condition of precipitation can also be affected by adding more of any of the precipitating ions. Example: If a solution is saturated with barium sulfate, Ba. SO 4(s) Ba 2+ + SO 42 -, Ksp = 9. 12 x 10 -11 If, more sulfate or Ba 2+ is added to the solution, the reaction will shift to the left ( more precipitate formed). This effect is called the common ion effect.

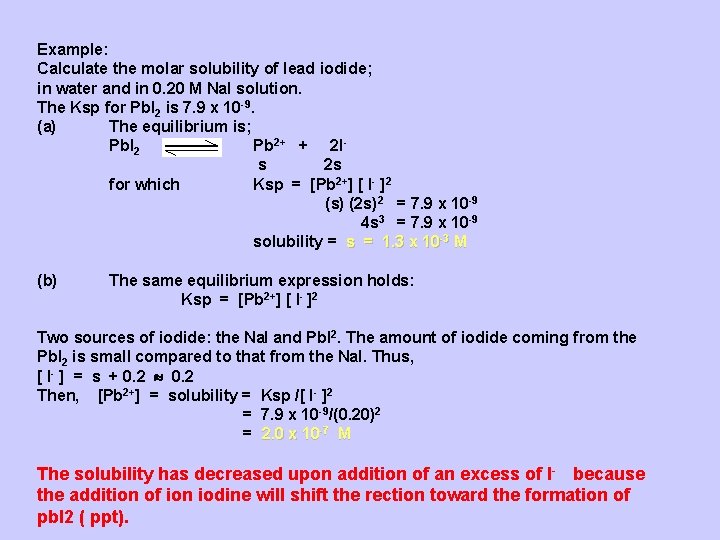
Example: Calculate the molar solubility of lead iodide; in water and in 0. 20 M Na. I solution. The Ksp for Pb. I 2 is 7. 9 x 10 -9. (a) The equilibrium is; Pb. I 2 Pb 2+ + 2 Is 2 s for which Ksp = [Pb 2+] [ I- ]2 (s) (2 s)2 = 7. 9 x 10 -9 4 s 3 = 7. 9 x 10 -9 solubility = s = 1. 3 x 10 -3 M (b) The same equilibrium expression holds: Ksp = [Pb 2+] [ I- ]2 Two sources of iodide: the Na. I and Pb. I 2. The amount of iodide coming from the Pb. I 2 is small compared to that from the Na. I. Thus, [ I- ] = s + 0. 2 Then, [Pb 2+] = solubility = Ksp /[ I- ]2 = 7. 9 x 10 -9/(0. 20)2 = 2. 0 x 10 -7 M The solubility has decreased upon addition of an excess of I - because the addition of ion iodine will shift the rection toward the formation of pb. I 2 ( ppt).

PRECIPITATING REAGENTS (PRECIPITANTS) • Precipitants are chosen for their ability to be selective and to form highly insoluble precipitates (easily filtered) and of reproducible stoichiometry. • Types of precipitant: inorganic and organic reagents. INORGANIC PRECIPITANTS • Form slightly soluble salts or hydrous oxides with the analyte. • Most inorganic reagents are not very selective. • Two common inorganic precipitating agents are, • silver nitrate, which is used to precipitate halide ions such as chloride, • barium chloride, which is used to precipitate sulfate ion.

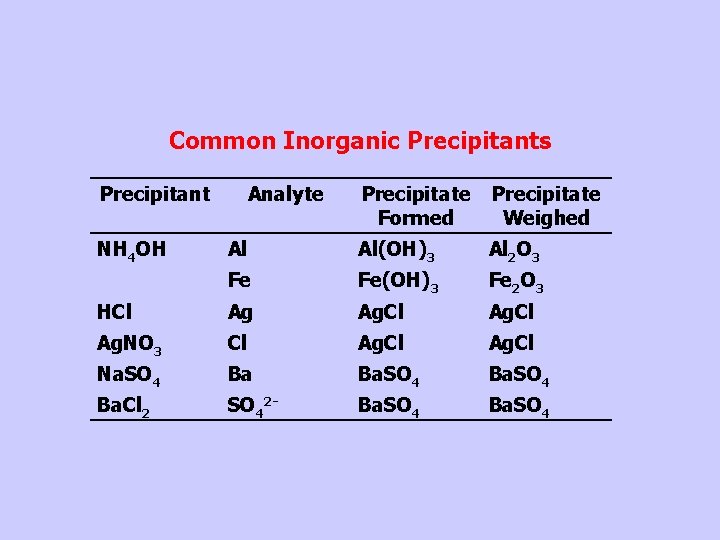
Common Inorganic Precipitants Precipitant Precipitate Formed Precipitate Weighed Al Al(OH)3 Al 2 O 3 Fe Fe(OH)3 Fe 2 O 3 HCl Ag Ag. Cl Ag. NO 3 Cl Ag. Cl Na. SO 4 Ba Ba. SO 4 Ba. Cl 2 SO 42 - Ba. SO 4 NH 4 OH Analyte

ORGANIC PRECIPITANTS • Very useful precipitating agents for metals. • Advantages by using organic reagents as a precipitant ; It forms chelate compounds with cations which are very insoluble in water. So, that metal ions may be quantitatively precipitated. • The organic precipitant often has a large molecular weight. Thus a small amount of metal may yield a large weight of precipitate. • Some of the organic reagents are fairly selective, yielding precipitates with only a limited number of cation. By controlling such factors as p. H and the concentration of masking reagents, the selectivity of an organic reagent can be enhanced. • The precipitates obtained with organic reagents are often coarse and bulky, and hence easily handle or filtered.

Examples: 8 - hydrixyquinoline (oxine) It can precipitates many elements but can be used for group separation by controlling p. H. Aluminium ion can be precipitated at p. H 4. A higher p. H is required to precipitate magnesium. Other organic precipitants: DMG – Ni 2+ and Co 2+ Cupferron – Fe 2+, Ce 4+ Nitron – NO 3 -, Cl. O 4 tetraphenyiarsonium chloride – Cr 2 O 72 -, Mn. O 4 -







