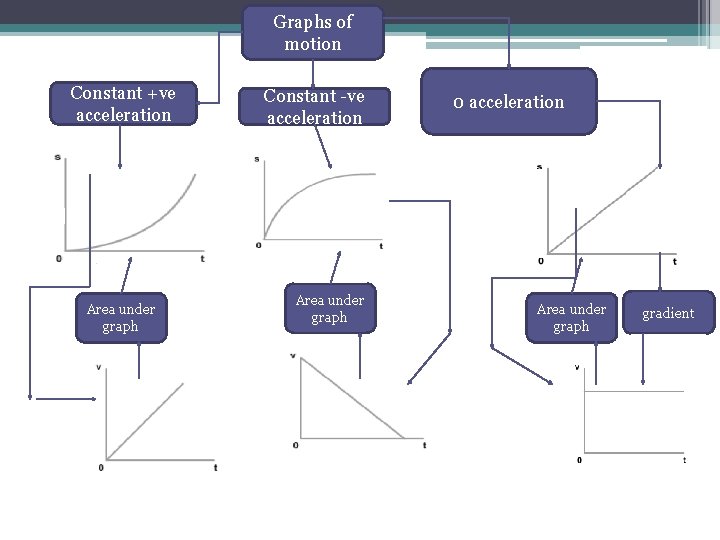
Graphs of motion Constant ve acceleration Area under
- Slides: 7

Graphs of motion Constant +ve acceleration Area under graph Constant -ve acceleration Area under graph 0 acceleration Area under graph gradient

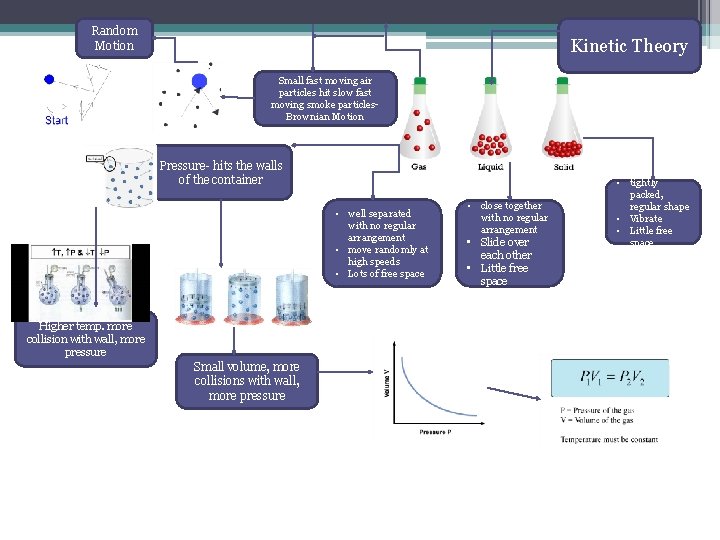
Random Motion Kinetic Theory Small fast moving air particles hit slow fast moving smoke particles. Brownian Motion Pressure- hits the walls of the container • well separated with no regular arrangement • move randomly at high speeds • Lots of free space Higher temp. more collision with wall, more pressure Small volume, more collisions with wall, more pressure • close together with no regular arrangement • Slide over each other • Little free space • tightly packed, regular shape • Vibrate • Little free space

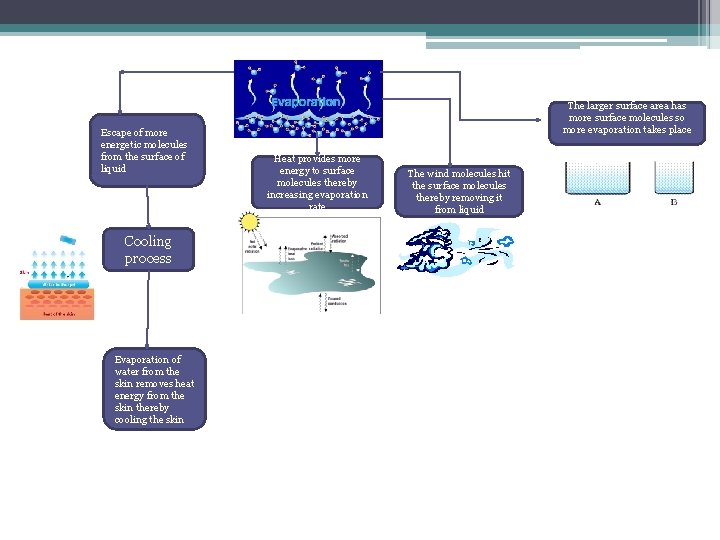
Escape of more energetic molecules from the surface of liquid Cooling process Evaporation of water from the skin removes heat energy from the skin thereby cooling the skin The larger surface area has more surface molecules so more evaporation takes place Heat provides more energy to surface molecules thereby increasing evaporation rate The wind molecules hit the surface molecules thereby removing it from liquid

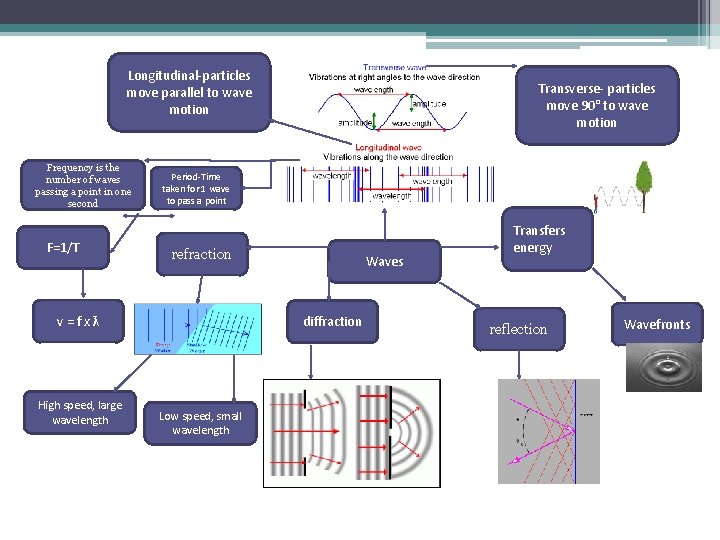
Longitudinal-particles move parallel to wave motion Frequency is the number of waves passing a point in one second F=1/T Period-Time taken for 1 wave to pass a point refraction v=fxƛ High speed, large wavelength Transverse- particles move 90° to wave motion Waves diffraction Low speed, small wavelength Transfers energy reflection Wavefronts

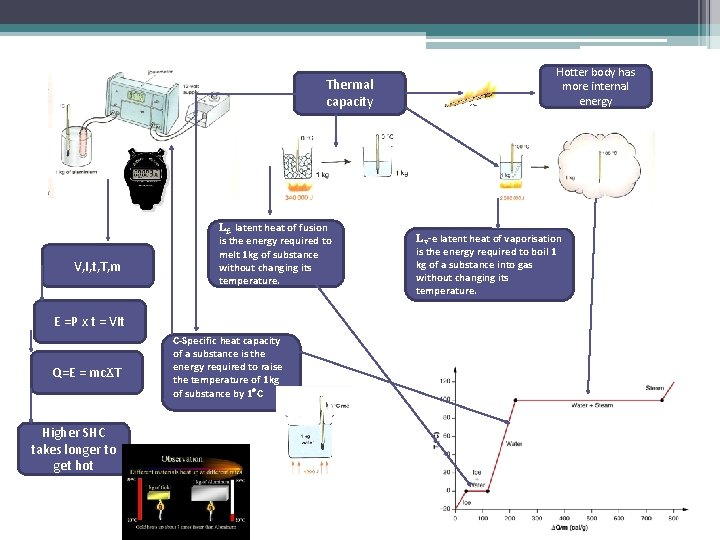
Thermal capacity V, I, t, T, m Lf- latent heat of fusion is the energy required to melt 1 kg of substance without changing its temperature. E =P x t = VIt Q=E = mcϪT Higher SHC takes longer to get hot C-Specific heat capacity of a substance is the energy required to raise the temperature of 1 kg of substance by 1 C Hotter body has more internal energy Lv-e latent heat of vaporisation is the energy required to boil 1 kg of a substance into gas without changing its temperature.

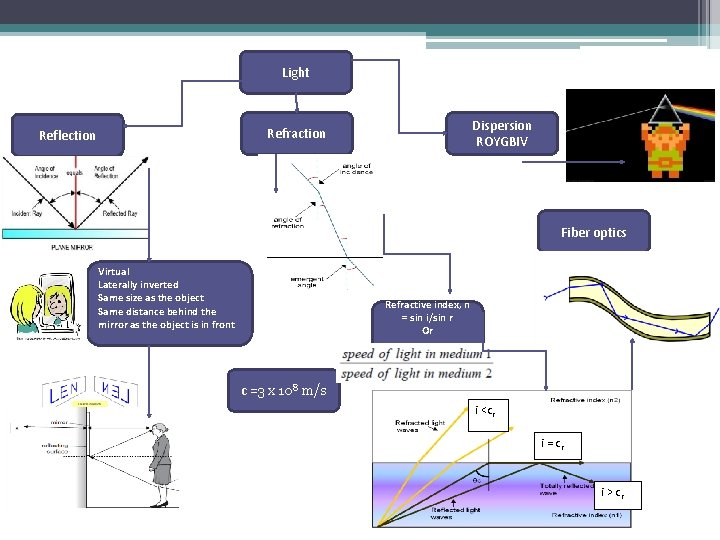
Light Dispersion ROYGBIV Refraction Reflection Fiber optics Virtual Laterally inverted Same size as the object Same distance behind the mirror as the object is in front Refractive index, n = sin i/sin r Or c =3 x 108 m/s i <cr i = cr i > cr

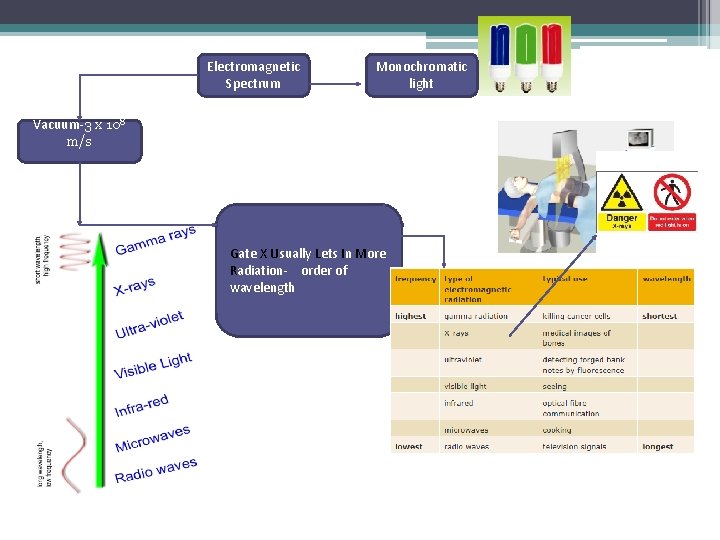
Electromagnetic Spectrum Monochromatic light Vacuum-3 x 108 m/s Gate X Usually Lets In More Radiation- order of wavelength