Gramnegative rods Enterobacteriaceae Characters of Enterobacteriaceae All Enterobacteriaciae

Gram-negative rods Enterobacteriaceae
Characters of Enterobacteriaceae All Enterobacteriaciae • Gram-negative rods • Ferment glucose with acid production • Reduce nitrates into nitrites • Oxidase negative Facultative anaerobic Motile except Shigella and Klebsiella Non-capsulated except Klebsiella Non-fastidious Grow on bile containing media (Mac. Conkey agar)
Enterobacteriaceae Some Enterobacteriaceae are true pathogens • Salmonella spp. • Shigella spp. • Yersinia spp. • Certain strains of E. coli (ETEC, EPEC, EIEC, EHEC) Most members of the Enterobacteriaceae are opportunistic or cause secondary infections of wounds, the urinary and respiratory tracts, and the circulatory system e. g. E. coli. Enterobacteriaceae divided into TWO main groups according to action on LACTOSE • Lactose Fermenters (LF) E. coli, Citrobacter, Klbesiella, Enterobacter • Lactose Non-Fermenters (LNF) Salmonella, Shigella, Proteus, Yersinia
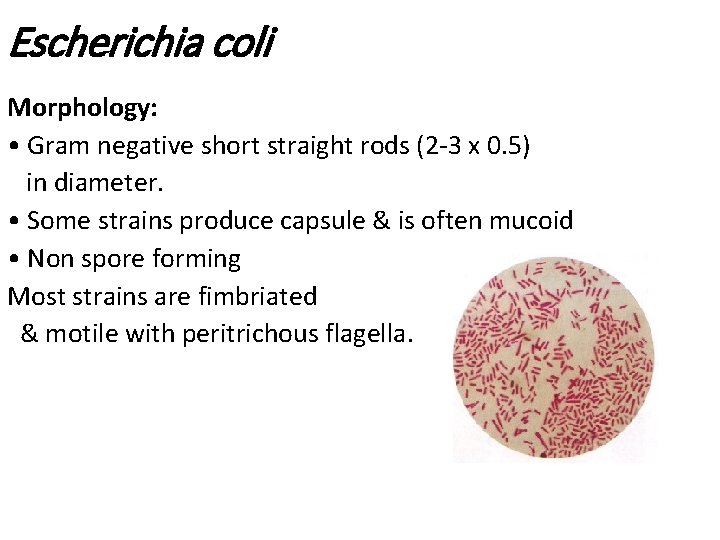
Escherichia coli Morphology: • Gram negative short straight rods (2 -3 x 0. 5) in diameter. • Some strains produce capsule & is often mucoid • Non spore forming Most strains are fimbriated & motile with peritrichous flagella.

Culture characteristics: • Facultative anaerobes & grow optimally at 37 °C. • Can grow on simple media producing circular, glistening unpigmented colonies. • On Mac. Conkeys agar media produce pink lactose fermenting colonies. • On EMB produce dark colonies with green metallic sheen. • Some stains are hemolytic, α and β hemolysis particularly those causing urinary tract infection.
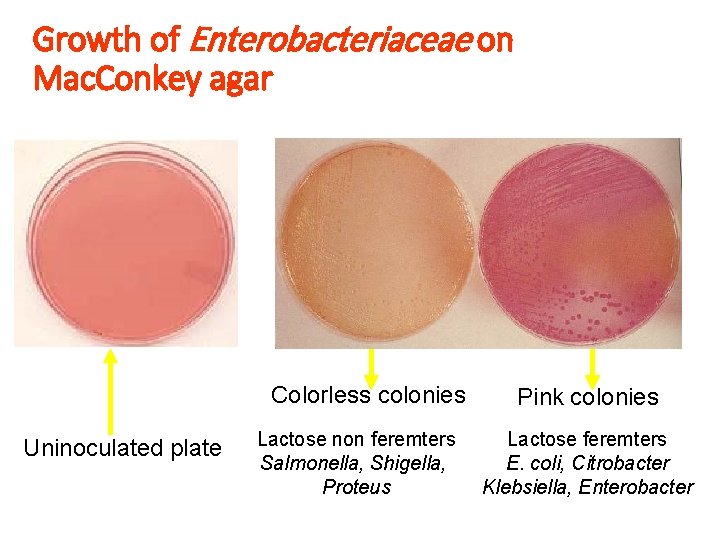
Growth of Enterobacteriaceae on Mac. Conkey agar Colorless colonies Uninoculated plate Lactose non feremters Salmonella, Shigella, Proteus Pink colonies Lactose feremters E. coli, Citrobacter Klebsiella, Enterobacter
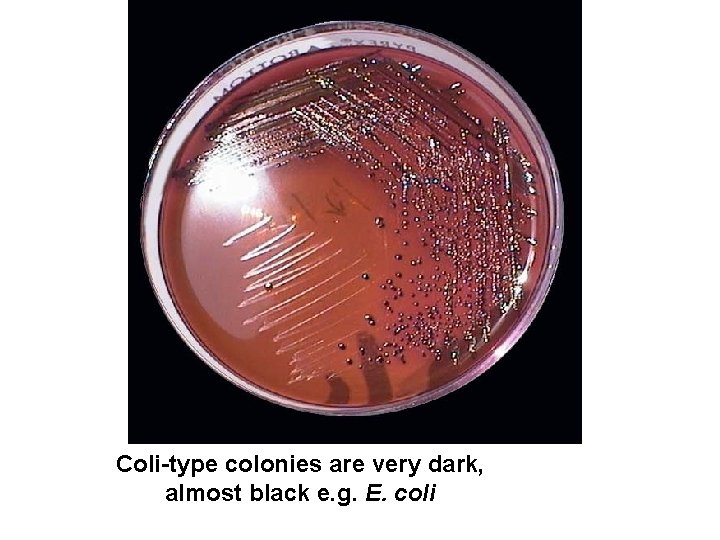
Coli-type colonies are very dark, almost black e. g. E. coli

Biochemical reaction butt acidic (yellow) Slant acidic (yellow) H 2 s production -ve E. Coli & Klebsiella
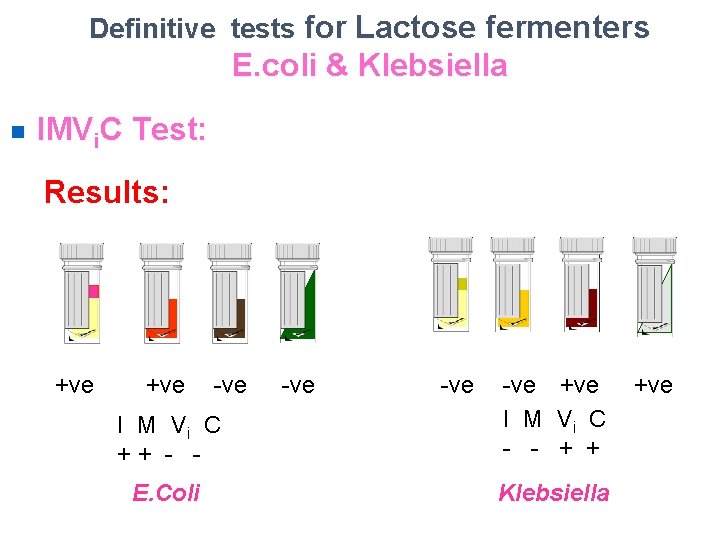
Definitive tests for Lactose fermenters E. coli & Klebsiella n IMVi. C Test: Results: +ve -ve I M Vi C + + - E. Coli -ve -ve +ve I M Vi C - - + + Klebsiella +ve
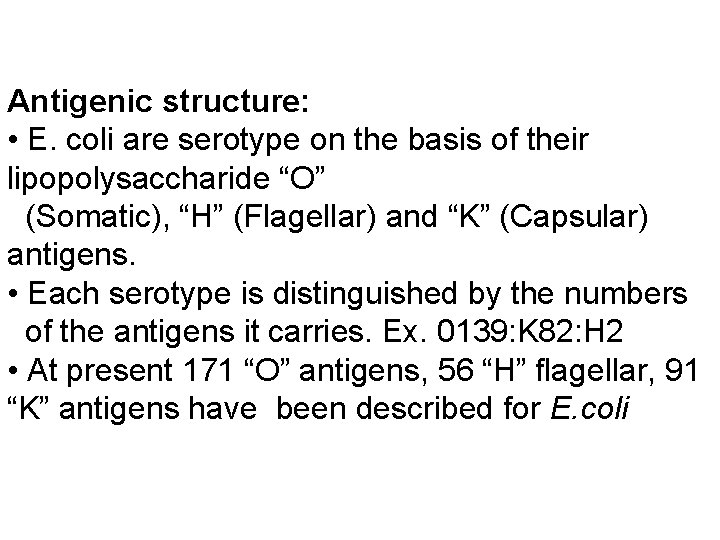
Antigenic structure: • E. coli are serotype on the basis of their lipopolysaccharide “O” (Somatic), “H” (Flagellar) and “K” (Capsular) antigens. • Each serotype is distinguished by the numbers of the antigens it carries. Ex. 0139: K 82: H 2 • At present 171 “O” antigens, 56 “H” flagellar, 91 “K” antigens have been described for E. coli

Virulence factors: 1 -Pili: which mediates adherance to mucosal surfaces 2 -K or capsular polysacharide Ag which is antiphagocytic 3 -LPS: which is responsible for the endotoxin manifestations. ’ 4 -Two exotoxins (they are enterotoxins) one is heat labile ( LT) and the second is heat stable (ST). 5 -The Enterohaemorrhagic stains of E-coli produce a Verotoxin or shiga toxin.
• The pathogenic strains: a) Uropathogenic E. coli colonize the vagina & periurethral region ascends to bladder or kidney causing cystitis & pyelonephritis They possess pilli that binds to specific receptors on the urinaryepithelium, K antigen & produce exotoxins (haemolysins ) b) Enteric E. coli:

Diseases caused by E. coli in different animals • E. coli has been isolated from cases of mastitis, urogenital infections. • E. coli causes a disease in young calves called white scours or colibacillosis characterized by greyish white diarrhea and sometimes with septicaemia which may cause death in few hours. • In horses, E. coli may be associated with other bacteria in causing a disease in foals known as joint ill or navel-ill. • Coli-granuloma or Hijarre's disease is a condition in poultry characterized by granulomatous lesions in the wall of the digestive tract and in the liver caused by E. coli infection.

Diagnosis: 1 -sample: According to the site of infection: ex. pus, urine, stool, csf. 2 -Film: ? 3 -Culture: ? 4 -B. R: ?

In case of diarrhoea: 1 -serotyping by slide agglutination for EPEC and EHEC 2 -when EHEC is suspected; rapid diagnostic methods are used to detect the verotoxin by ELISA, or to detect the organism by immunofluorescence in stool. 3 -PCR, DNA probes or tissue cultures can be used to detect the toxin genes or toxin effects.
Salmonella
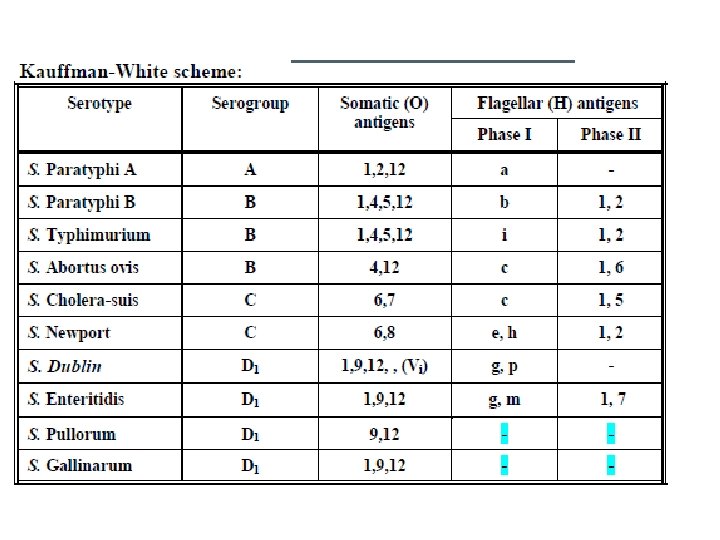
Salmonella are recently classified according to DNA-DN hybridization into 7 groups. Nearly all of the Salmonella serotypes that infect humans are in group I which includes more than 1400 serotypes. Salmonella enterica is the most important species.

Morphology: Gram negative bacilli (0. 7 - 1. 5 x 2 - 5 µm) Nonspore forming, motile with peritrichous flagella except S. pullorum & S. gallinarum Culture characteristics: - Facultative anaerobes, grow well on ordinary media produce smooth colonies 2 -3 mm in diameter. On Mac. Conkey's agar media it produces pale non lactose fermenting colonies. On Bismuth sulphate agar produces black colonies
Enterobacteriaceae ii) lactose non-fermenters (NLFS) Salmonella Shigella Proteus (urease +ve)
Biochemical characteristics Do not ferment lactose but ferment glucose, maltose and mannitol with production of acid and gas. Produce H 2 S, reduces nitrates & gelatin is rarely liquefied. Indol, urease & V. P. negative M. R citrate usually +ve
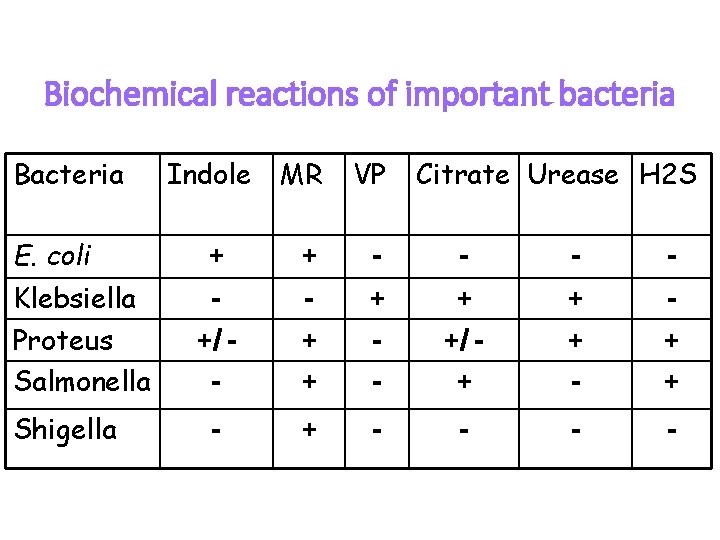
Biochemical reactions of important bacteria Bacteria E. coli Klebsiella Proteus Salmonella Shigella Indole MR VP Citrate Urease H 2 S + + - - +/ - + + + - + +/ + + + - + - -
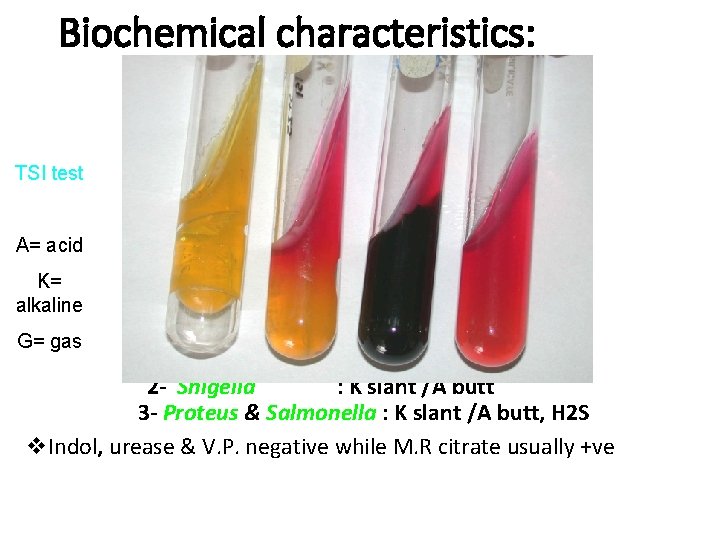
Biochemical characteristics: TSI test A= acid K= alkaline G= gas 1 - Escherichia coli : A slant/ A butt +ve 2 - Shigella : K slant /A butt 3 - Proteus & Salmonella : K slant /A butt, H 2 S v. Indol, urease & V. P. negative while M. R citrate usually +ve

From the epidemiological point of view, Salmonella can be classified into 3 main groups • Group 1: Those infecting only humans Ex. S. typhi, Paratyphi A and C • Group 2: Those adapted for particular species of vertebrates Ex. S. gallinarum for poultry, S. dublin in cattle, S. abortus equi in horses, S. abortus ovis in sheep, S. choleraesuis in swine. • Group 3: Salmonella types with no particular host preferences. • Infect both humans and animals (PARATYPHOIDS)

Diseases produced by Salmonella species of veterinary importance I) Poultry: - Etiology of salmonellosis in poultry: S. pullorum disease • Affects mainly 1 - 14 day old chicks • Causes severe outbreaks mortality rate 95 - 100 % • Characterized clinically by white diarrhea & sudden death. • Forms symptomless carrier & the organism localized in ovaries. • Eggs layed by infected birds contain S. pullorum in yolk.
S. gallinarum fowel typhoid • Affects mainly adult flocks. • Route of infection is via ingestion of contaminated food & water. • Causes 50 % mortality. • Characterized clinically with greenish diarrhea.

II) Cattle: • Etiology of bovine salmonellosis S. dublin, S. typhi-murium, S. newport & S. abortus bovis. • They affect mainly young calves causing diarrhoea, loss of appetite, weakness and death in a few days with blood-stained mucoid feaces • Pregnant animals may abort.

III) Sheep and goats: Etiology of ovine salmonellosis S. dublin and S. typhi-murium which cause enteritis. Outbreaks of abortion are caused by S. abortus ovis especially during the last two months of pregnancy. IV) Horses: Etiology of ovine salmonellosis S. abor equi which causes abortions in pregnant equines during the last two months of pregnancy. Newborn foals may be infected with diarrhea, septicemia and death may occur within 2 -3 days later

Diagnosis • Sample: 1 - In early stage of the disease blood especially in septicemic form or in case of enteric fever, later the organism is found in stool & urine. 2 - Smear is of no value due to the presence of large number of normal intestinal 3 - Stool samples must be inoculated into enrichment media as tetrathionate broth or selenite F broth. 4 - Isolation & identification.
5 - Make film from the isolated colonies stained with Gram stain Gram gram neg bacilli. 6 - Phage typing: for epidemiological studies to identify the isolates 7 - Serological identification of the isolates Slides or tube agglutination tests is used for the detection of infected living birds or animals. a) Slide (rapid) agglutination test: Mix one drop of fresh whole blood with one drop of coloured stained antigen: clumping (pos. ) b) Tube (slow) agglutination test may be used to determine the titre of the antibodies. ELISA & immuno fluorescence can be used 8 - Molecular identification using PCR
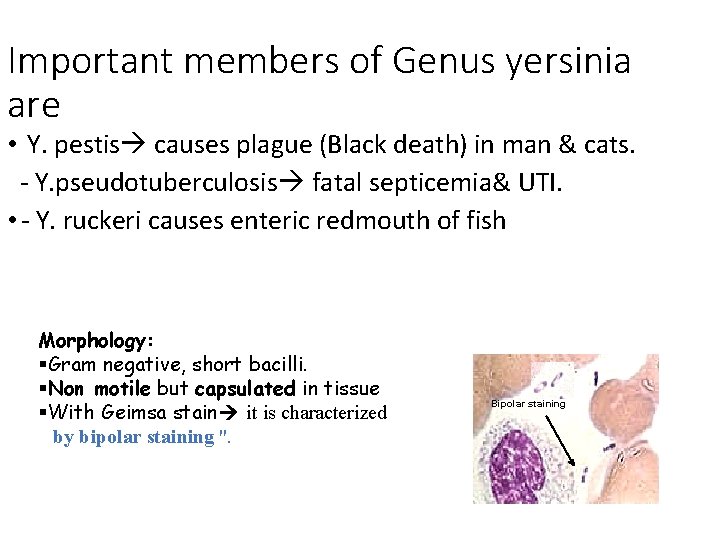
Important members of Genus yersinia are • Y. pestis causes plague (Black death) in man & cats. - Y. pseudotuberculosis fatal septicemia& UTI. • - Y. ruckeri causes enteric redmouth of fish Morphology: Gram negative, short bacilli. Non motile but capsulated in tissue With Geimsa stain it is characterized by bipolar staining ''. Bipolar staining

Virulence factors: • V & W antigens of Y. pestis cell walls which are protein-lipoprotein complexes that prevent phagocytosis. • F 1 antigen antiphagocytic. • Yops (yersinia outer proteins) antiphagocytic, inhibit cytokines production ability of bacteria to invade & multiply in host cells. • Endotoxin (Lps) endotoxic shock.

Disease produced: • Y. pestis causes plague in man & cat • Plague is a zoonotic disease of rodents that affects man & cats & caused by the bite of rat flea • Plague has 3 forms (Bubonic –> Pneumonic-> Septicemic form
Yersinia enterocolitica • • Foodborne pathogen causes yersiniosis • • Grows at wide range of temperatures (0 - 44 °C), aerobically & anaerobically. • Withstands freezing & survives in damp soil but destroyed by pasteurization. • • Pathogenesis: it adheres & penetrates terminal ileum epithelial cells invades intestinal mucosa multiplies in lymphoid tissue entritis. • • Sources of infection: for animals primary source is pigs rats. Insects.

Yersinia pseudotuberculosis • • Primarily an animal pathogen, occasionally causes disease in humans • • Affects rodents, turkeys, goats, swine, cattle, sheep, wild birds &man. • • Causes fever & acute mesenteric lymphadenitis in humans. • • Causes fatal septicemia & urinary tract disturbances in animals
Diagnosis: • 1 - Sample: blood, lymph node aspirate, stool • 2 - Film stained with Giemsa stain see morphology. • 3 - Isolation & identification see culture characteristics & biochemical reactions. • 4 - Laboratory animal inoculation: • I/M or S/C injection of the samples into mice or g. pigs septicemia & death the organism can be isolated from liver & spleen.
• 5 - Cold enrichment improves the isolation of the organism from stool samples. Procedure: • A suspension of 5 % stool sample in phosphate buffer saline is held at 4°C for 3 weeks and subcultured weekly on Mac. Conkey's agar under aerobic conditions. • 6 - Recent diagnostic methods as ELISA & PCR are useful.
- Slides: 37