Grahams Law Bromfield Honors Chemistry Diffusion and Effusion
Graham’s Law Bromfield Honors Chemistry

Diffusion and Effusion • Diffusion – The gradual mixing of 2 gases due to random spontaneous motion
Diffusion and Effusion • Effusion – When molecules of a confined gas escape through a tiny opening in a container

Graham’s Law • Thomas Graham (1805 -1869) • Do all gases diffuse at the same rate? • Graham’s law discusses this quantitatively. • Technically, this law only applies to gases effusing into a vacuum or into each other.
Graham’s Law • Conceptual: – At the same temperature, molecules that are less massive travel at a faster speed than molecules that are more massive • As molar mass , v

Graham’s Law • Consider H 2 vs. Cl 2 Which would diffuse at the greater velocity?

Applications of Graham’s Law • Separation of uranium isotopes – 235 U – Simple, inexpensive technique – Used in Iraq in early 1990’s as part of nuclear weapons development program
Applications of Graham’s Law • Identifying unknowns – Use relative rates to find molar mass
Graham’s Law • The relative rates of diffusion of two gases vary inversely with the square roots of the molar masses.
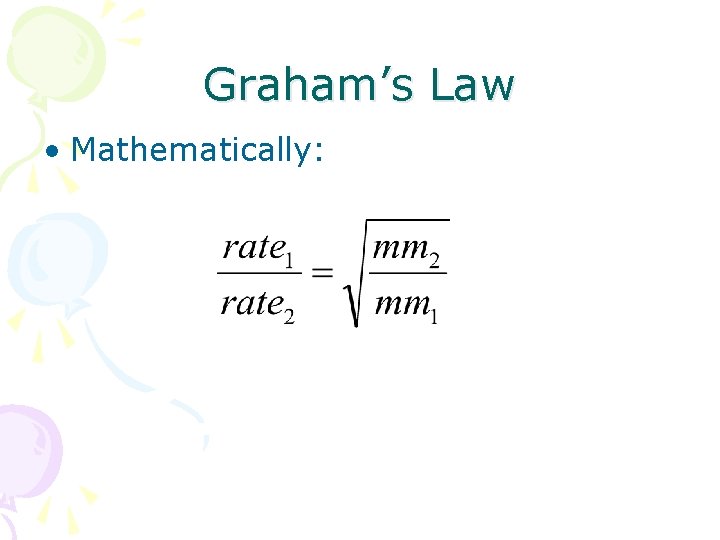
Graham’s Law • Mathematically:

Graham’s Law Problem • A helium atom travels an average 1000. m/s at 250 o. C. How fast would an atom of radon travel at the same temperature?
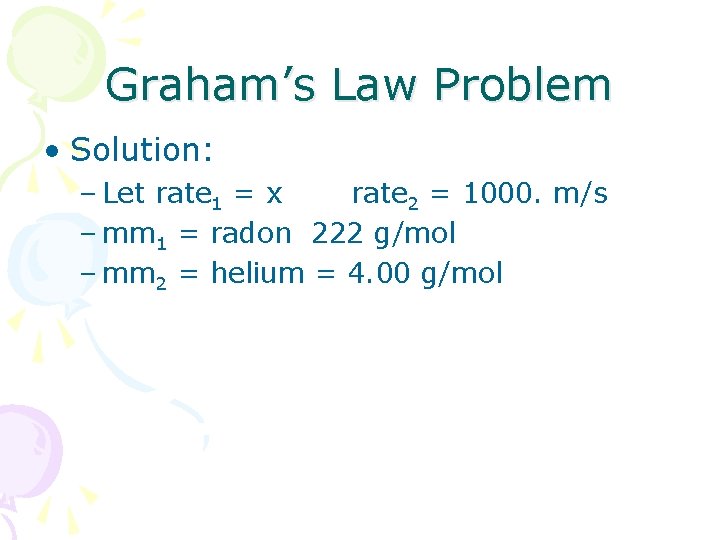
Graham’s Law Problem • Solution: – Let rate 1 = x rate 2 = 1000. m/s – mm 1 = radon 222 g/mol – mm 2 = helium = 4. 00 g/mol
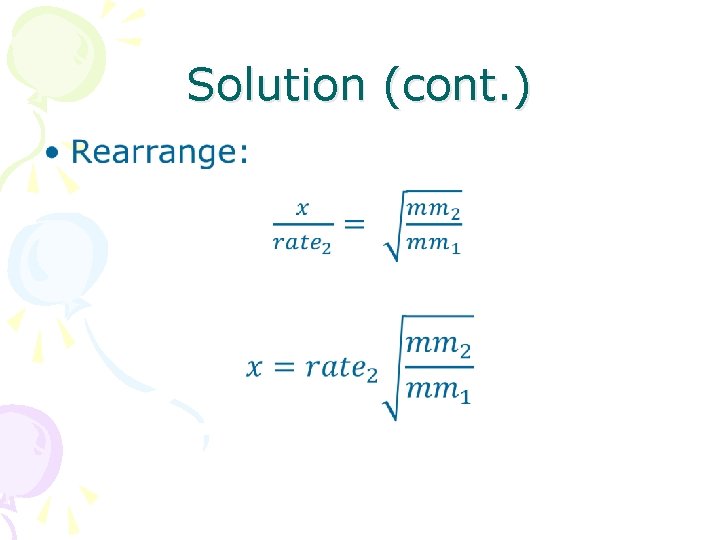
Solution (cont. ) •
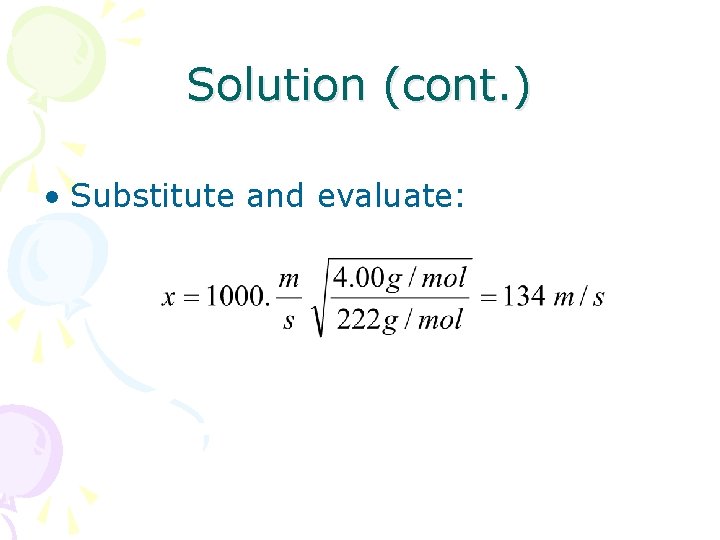
Solution (cont. ) • Substitute and evaluate:

Problem 2 • An unknown gas effuses through an opening at a rate 3. 16 times slower than that of helium gas. What is the molar mass of this unknown gas?
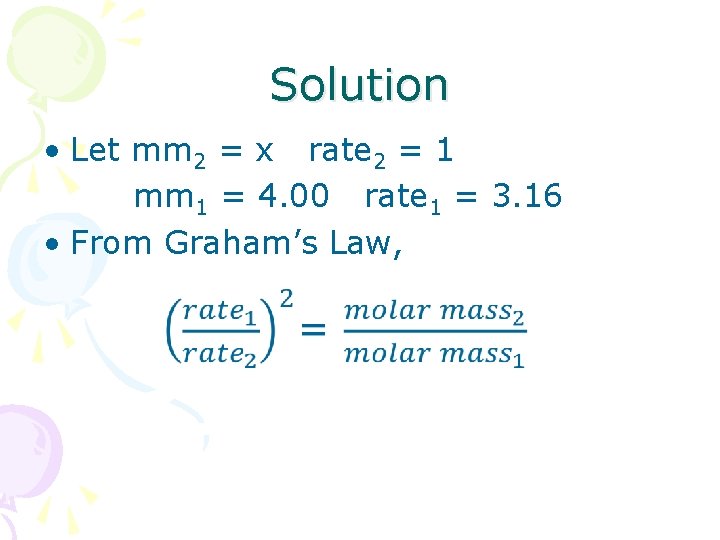
Solution • Let mm 2 = x rate 2 = 1 mm 1 = 4. 00 rate 1 = 3. 16 • From Graham’s Law,
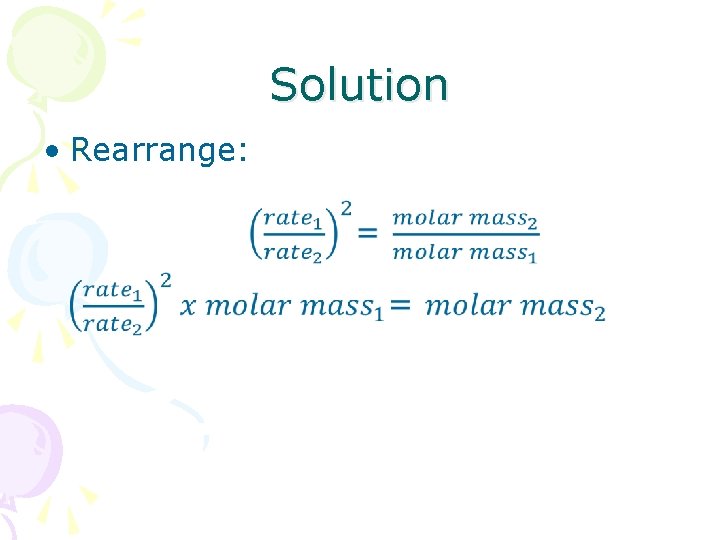
Solution • Rearrange:
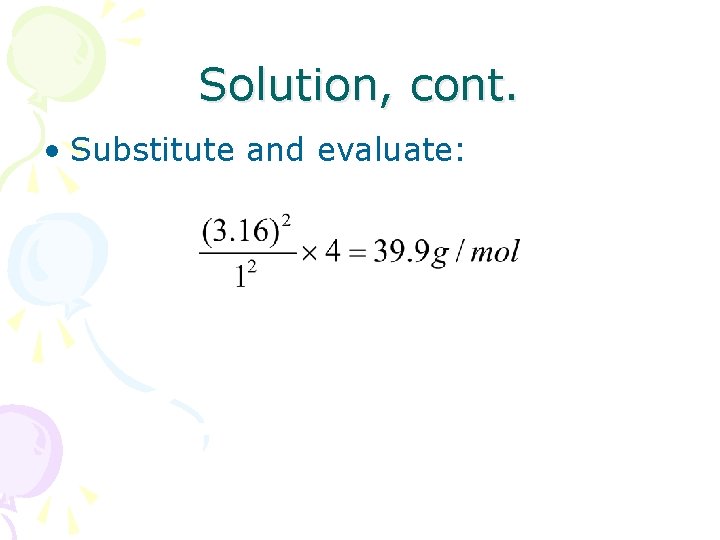
Solution, cont. • Substitute and evaluate:
- Slides: 18