Grade 9 Science Unit 1 Atoms Elements and

















- Slides: 33

Grade 9 Science Unit 1: Atoms, Elements, and Compounds

Grade 9 Science. . . Unit 1 Chapter 1: Atomic theory explains the composition and behaviour of matter.

Lab Safety. . . A Review �Safety MUST be your top priority. �Know them before you do the lab and use them while doing the lab.

Laboratory Complete activity 1 -1 A pg. 9 Safety

Safety Rules for the Science Lab pages 10 -11 �General �Glassware �Chemicals �Hot plates and open flames �Electrical equipment

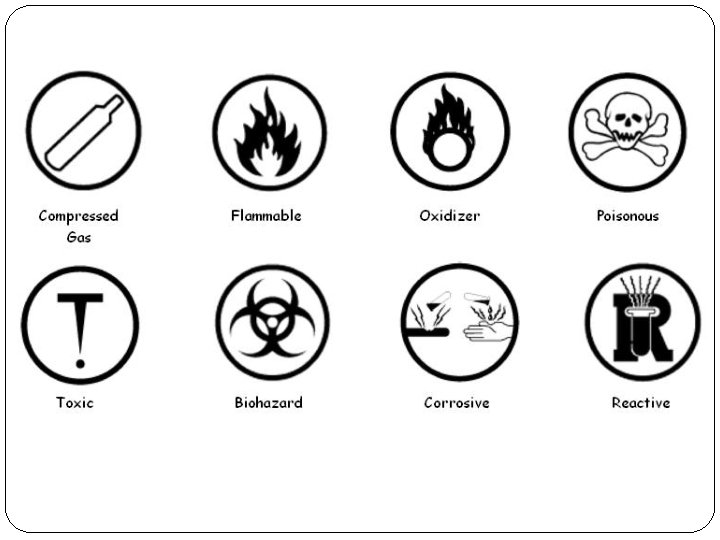
WHMIS. . . Page 12 W H M I S workplace hazardous materials information system


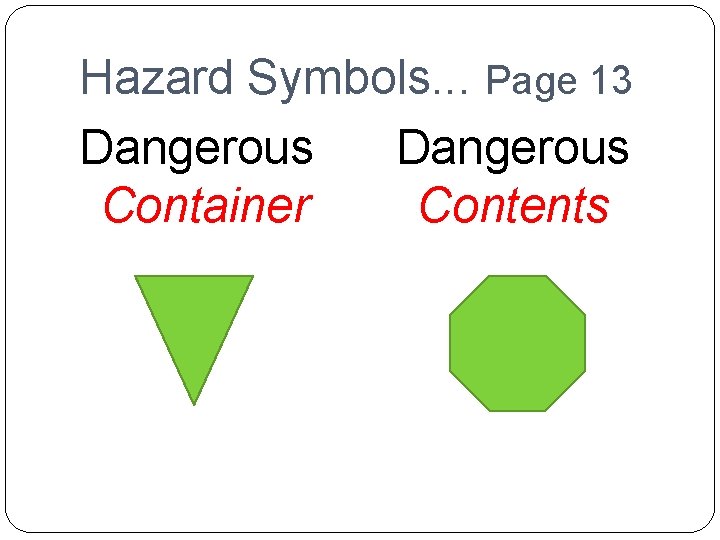
Hazard Symbols. . . Page 13 Dangerous Container Contents

Properties of Matter �Matter is anything that has mass and volume. �Mass is the amount of matter in a substance or object. �Volume is the amount of space a substance or object occupies.

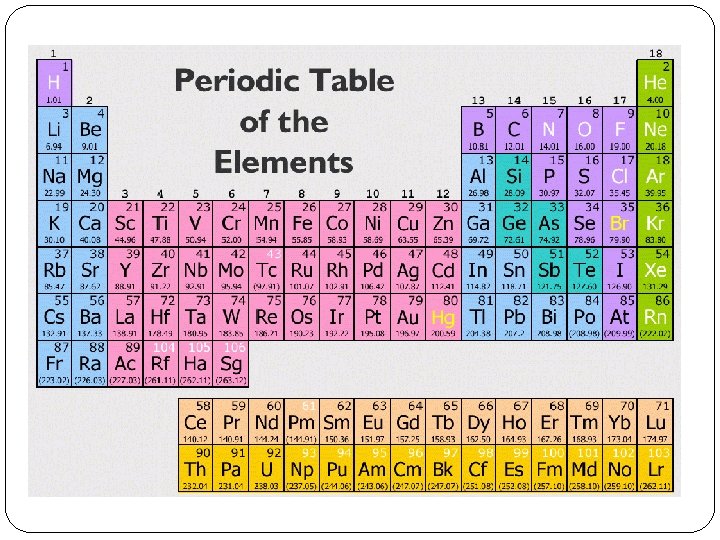
�Matter is made up of elements. �Elements are substances that contain one type of matter and cannot be broken down or separated into simpler substances.


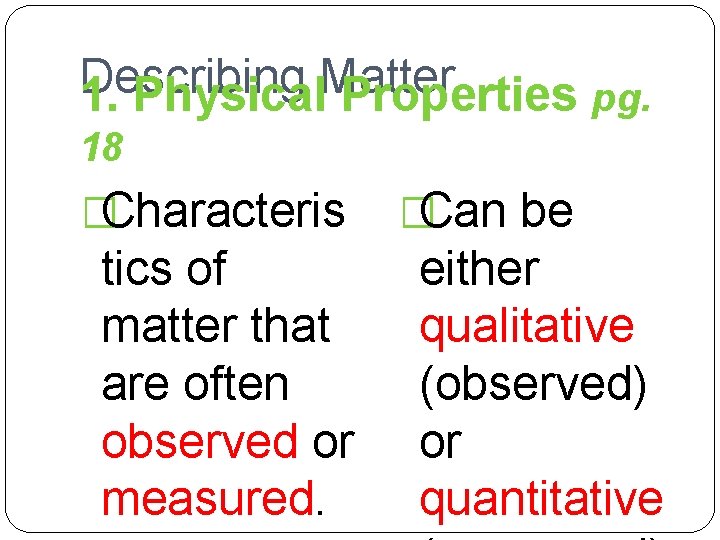
Describing Matter 1. Physical Properties pg. 18 �Characteris tics of matter that are often observed or measured. �Can be either qualitative (observed) or quantitative

• Color • Malleability • Lustre • Conductivity • Boiling point • Melting point • Texture • Magnetism

2. Chemical Properties pg. 19 �Observed �Determines a substances when substanc usefulness. es react with each other.

• Reactivity • The degree to which substances combine to form new compounds • Combustibility • The degree to which substances combine with oxygen (burn) • Toxicity • The degree to which a chemical is poisonous to living things

Core Lab Activity 1 -2 C pg. 20 Physical and Chemical

Theory vs. Law �A theory is less well supported than a law. �Theories try to explain observations �Examples: � Theory of Evolution � Theory of plate tectonics

Laws �Most laws are supported by different and robust experimental evidence. �Laws only tell what happens, not why it happens �Examples: �Law of Gravity �Newtons Laws of Motion

Atomic Theory �The descriptions of matter and how it behaves. �Has undergone many modifications as new facts became available.

Early ideas. . . 2000 years ago �Empedocles: matter was composed of four “elements”; earth, air, water, and fire.

�Democritus: eventually a substance will be cut into a piece that can no longer be cut. He called this piece atomos.

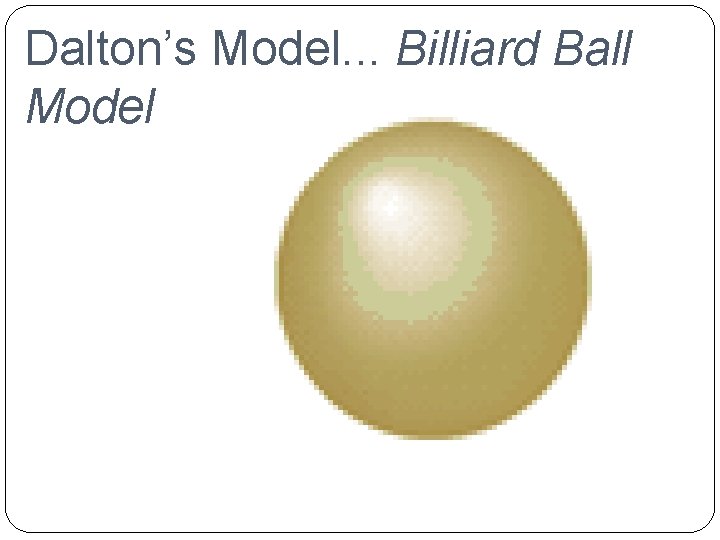
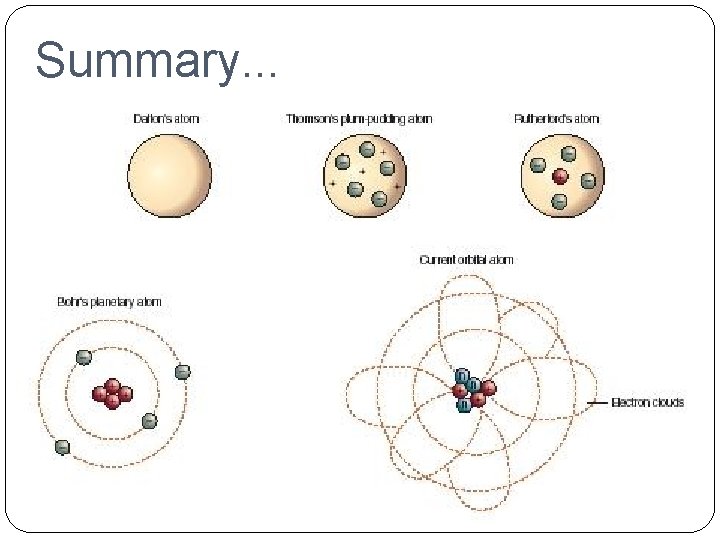
Development of Atomic Theory �John Dalton (1766 -1844) �Billiard Ball Model He suggested that the particles that make up matter are like small, hard spheres that are different for different elements. He defined an atom as the smallest particle of an element.

Dalton’s Model. . . Billiard Ball Model

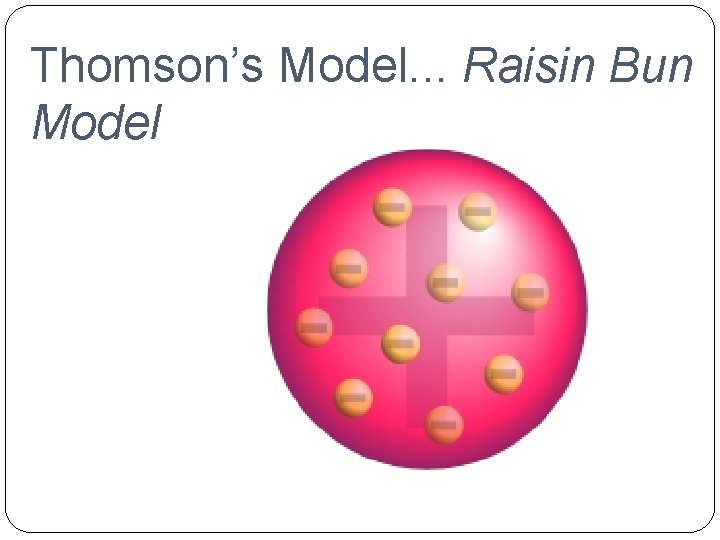
�J. J. Thomson (1856 -1940) �Raisin Bun Model He suggested that all atoms must contain electrons (negative charge). His model pictured a positively charged ball with the negatively charged electrons embedded in it.

Thomson’s Model. . . Raisin Bun Model

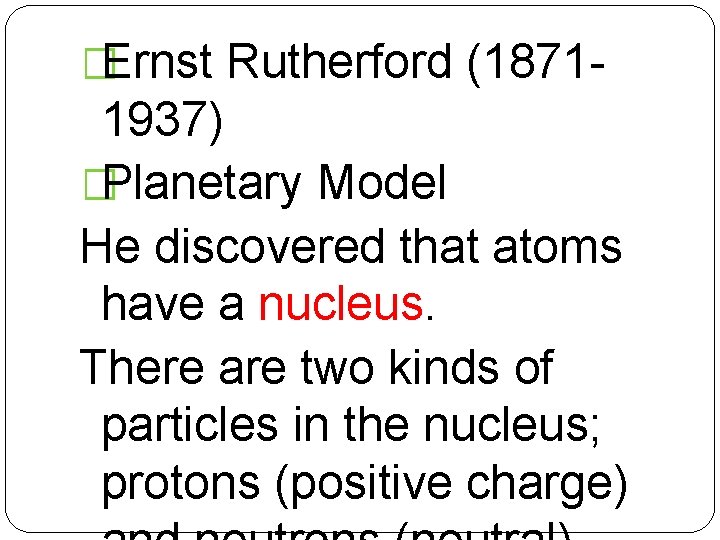
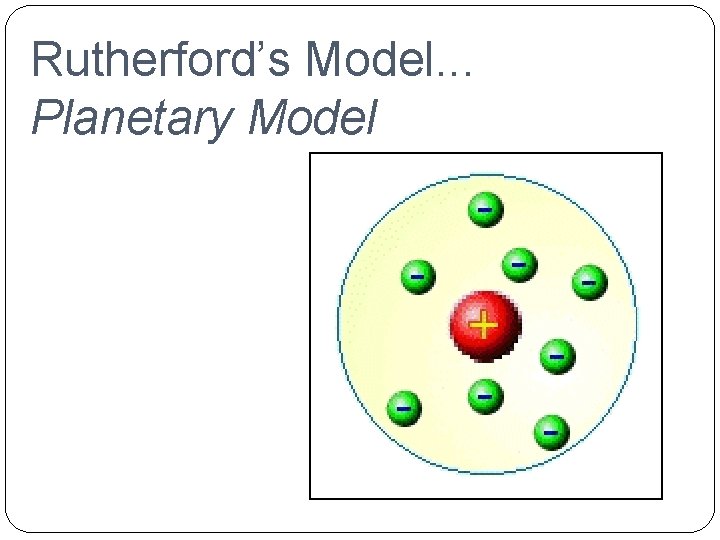
�Ernst Rutherford (1871 - 1937) �Planetary Model He discovered that atoms have a nucleus. There are two kinds of particles in the nucleus; protons (positive charge)

Rutherford’s Model. . . Planetary Model

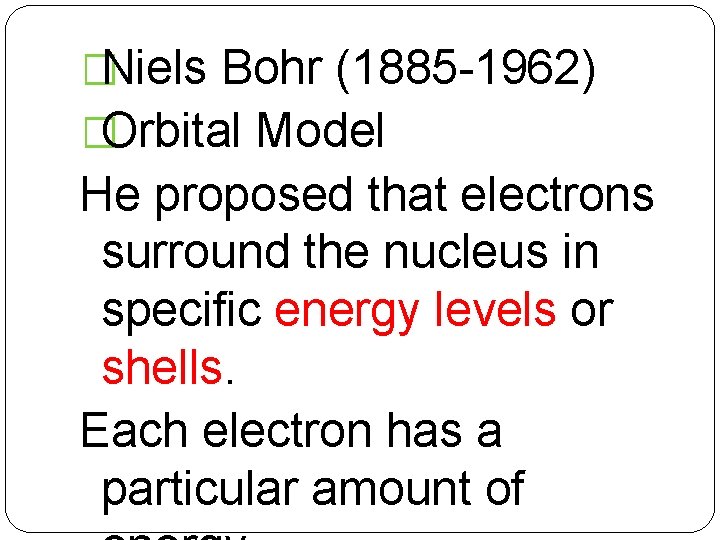
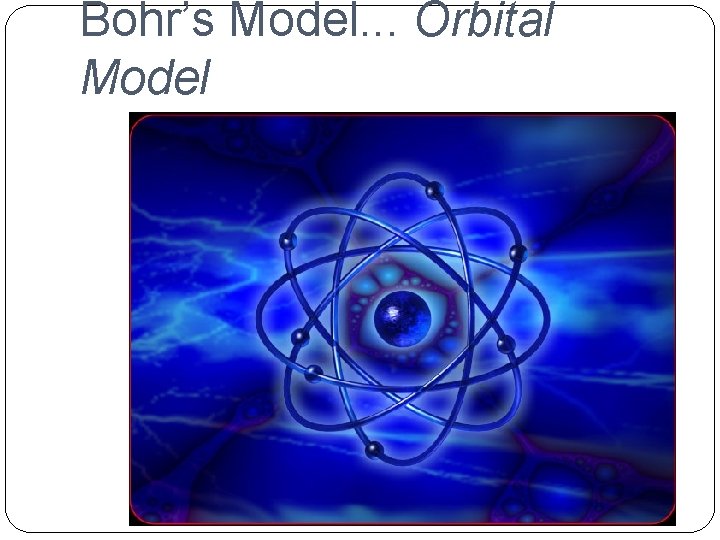
�Niels Bohr (1885 -1962) �Orbital Model He proposed that electrons surround the nucleus in specific energy levels or shells. Each electron has a particular amount of

Bohr’s Model. . . Orbital Model

• Rutherford was able to develop Thomson’s model due to the development of new technologies. (gold foil experiment) • The development of cyclotrons and proton accelerators have further developed the model

Summary. . .

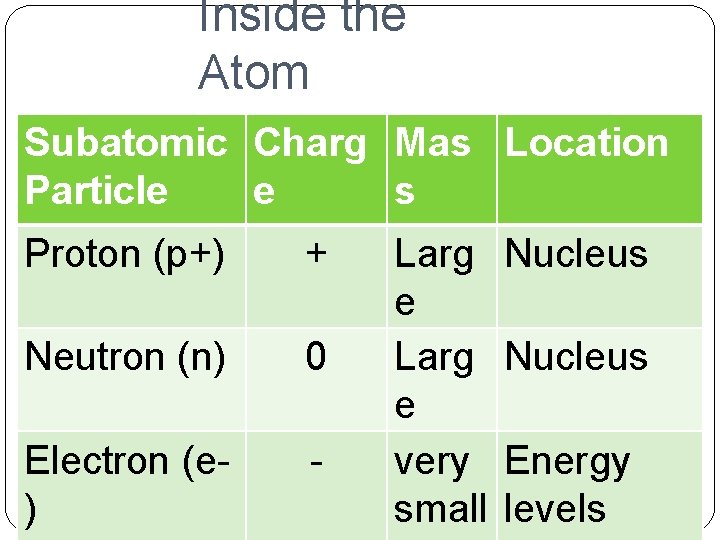
Inside the Atom Subatomic Charg Mas Location Particle e s Proton (p+) + Neutron (n) 0 Electron (e) - Larg e very small Nucleus Energy levels

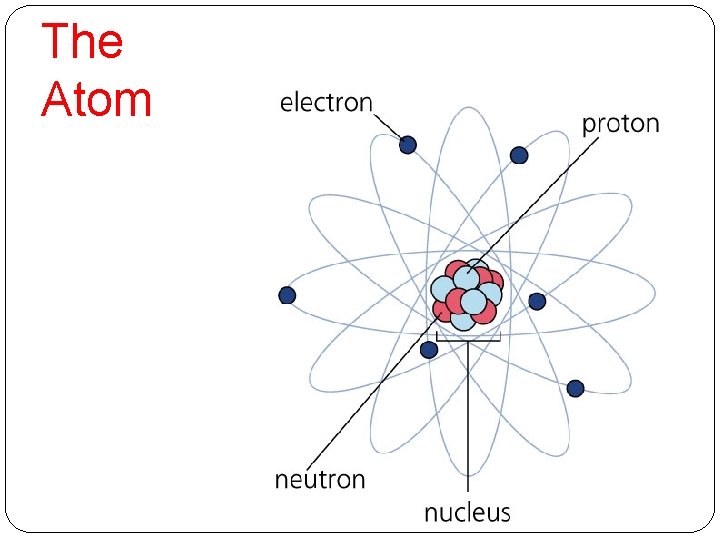
The Atom