Grade 7 Science Unit 3 Matter can be
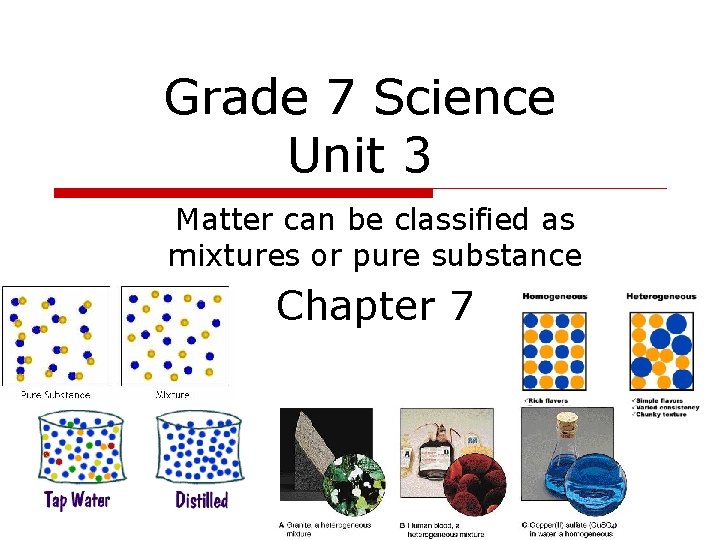
Grade 7 Science Unit 3 Matter can be classified as mixtures or pure substance Chapter 7

The Story of Archimedes *Read Story 228 -229

Review: Particle Theory of Matter 1. All matter is made up of tiny particles. 1. These particles are always moving. . . they have energy 1. There are spaces among particles. 1. There attractive forces between the particles. 1. The particles of one substance differ from the particles of other
Mini-Partner Activity 1 Together think of examples for each, then answer the following 2 questions. Pure Substance Mixture

1. How would you tell the difference between a pure substance and a mixture? ______________________________ ______________________________

1. How would you tell the difference between a pure substance and a mixture? __See (the properties) if you can see the different parts, (different states, different_ colour, texture, etc). A pure substance should be the samethroughout______
2. How would use the Particle Theory of Matter to support your answer to question #1? ______________________________________________
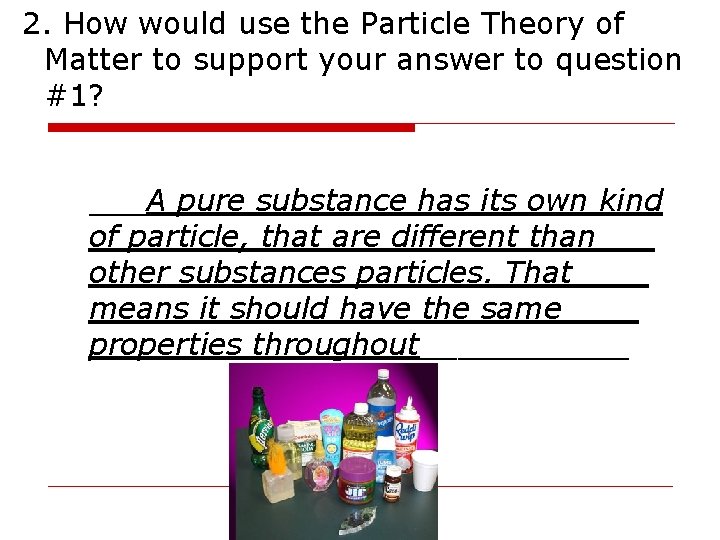
2. How would use the Particle Theory of Matter to support your answer to question #1? ___A pure substance has its own kind of particle, that are different than___ other substances particles. That____ means it should have the same____ properties throughout______

Mixtures Vs. Pure Substances How many visible Parts? Mixtures Pure Substances May have more than Have ONLY one visible part (not visible part always - think salt/sugar water)
Mixtures Vs. Pure Substances Uniform Througout? Mixtures Pure Substances MAY appear ALWAYS appear as uniform throughout
Mixtures Vs. Pure Substances Definition? Mixtures They are the physical combination of two or more pure substances. Pure Substances They contain either a single particle or two or more atoms chemically combined to form a different substance.
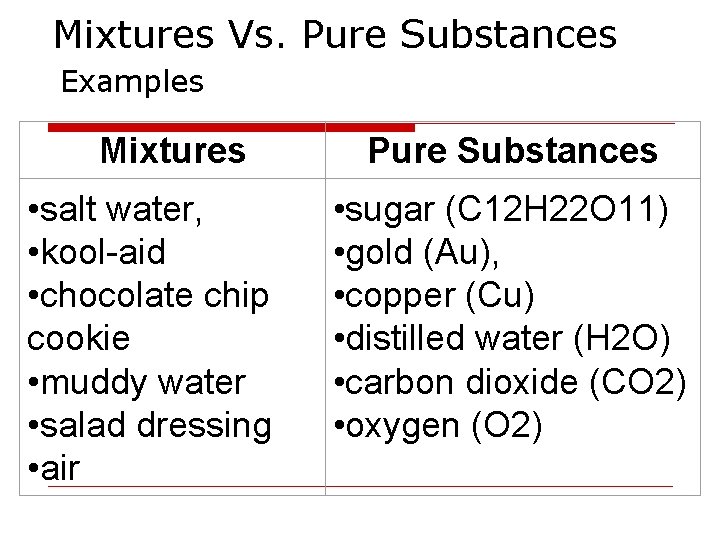
Mixtures Vs. Pure Substances Examples Mixtures • salt water, • kool-aid • chocolate chip cookie • muddy water • salad dressing • air Pure Substances • sugar (C 12 H 22 O 11) • gold (Au), • copper (Cu) • distilled water (H 2 O) • carbon dioxide (CO 2) • oxygen (O 2)
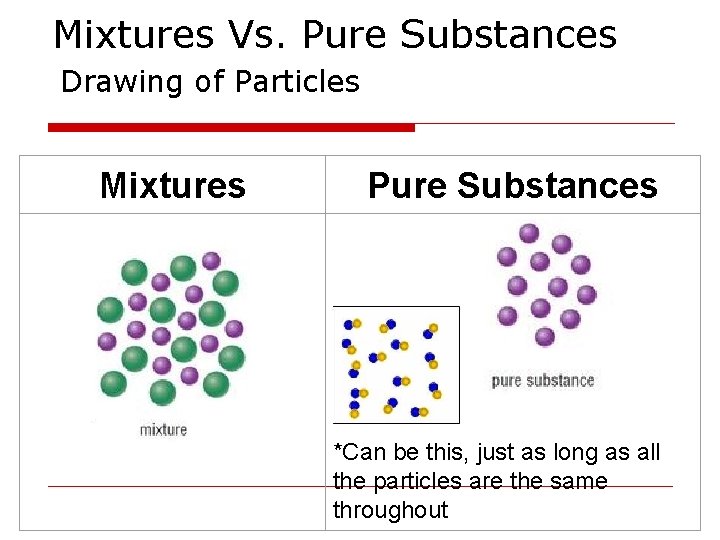
Mixtures Vs. Pure Substances Drawing of Particles Mixtures Pure Substances *Can be this, just as long as all the particles are the same throughout

Mini-Partner Activity 2 Read pages 232, 236, and 237. Make a list of 15 -20 solutions and mixtures that you encounter in a day. Put a * those that may pose a safety risk.
Soltions Vs. Mixtures! Solutions Mixtures

Practice Questions (Homework) Pg 237 Q’s 1, 2 &3
Read Page 242 - Complete the Following Flow Chart
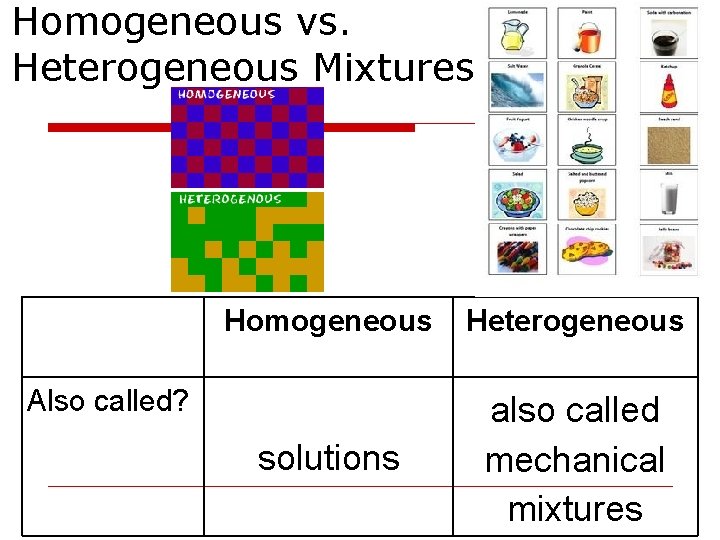
Homogeneous vs. Heterogeneous Mixtures Homogeneous Heterogeneous solutions also called mechanical mixtures Also called?
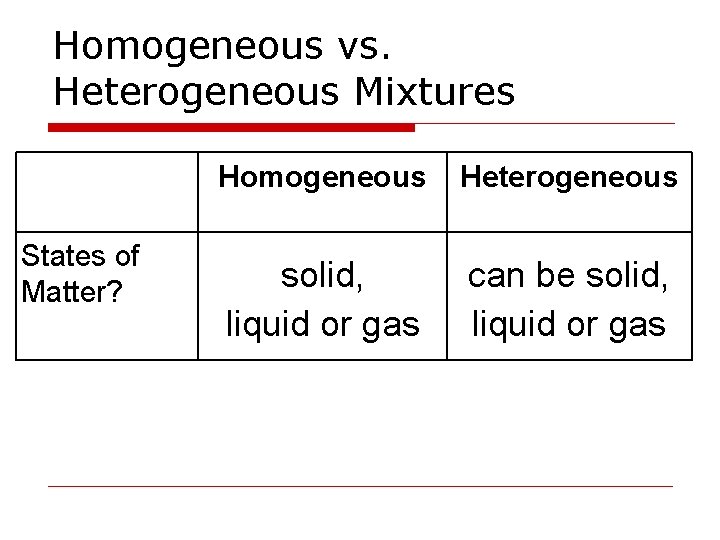
Homogeneous vs. Heterogeneous Mixtures States of Matter? Homogeneous Heterogeneous solid, liquid or gas can be solid, liquid or gas
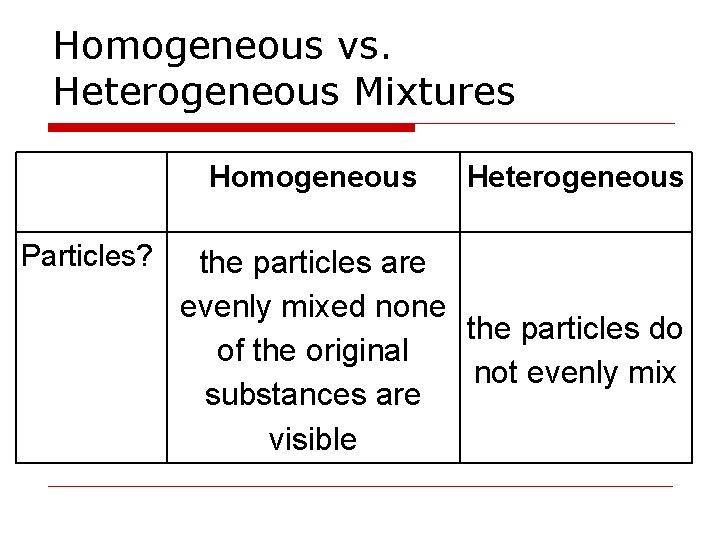
Homogeneous vs. Heterogeneous Mixtures Homogeneous Particles? Heterogeneous the particles are evenly mixed none the particles do of the original not evenly mix substances are visible
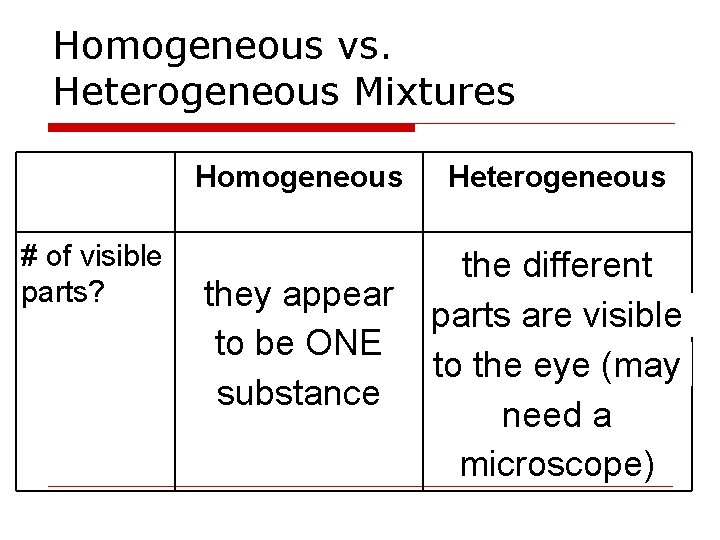
Homogeneous vs. Heterogeneous Mixtures Homogeneous # of visible parts? they appear to be ONE substance Heterogeneous the different parts are visible to the eye (may need a microscope)
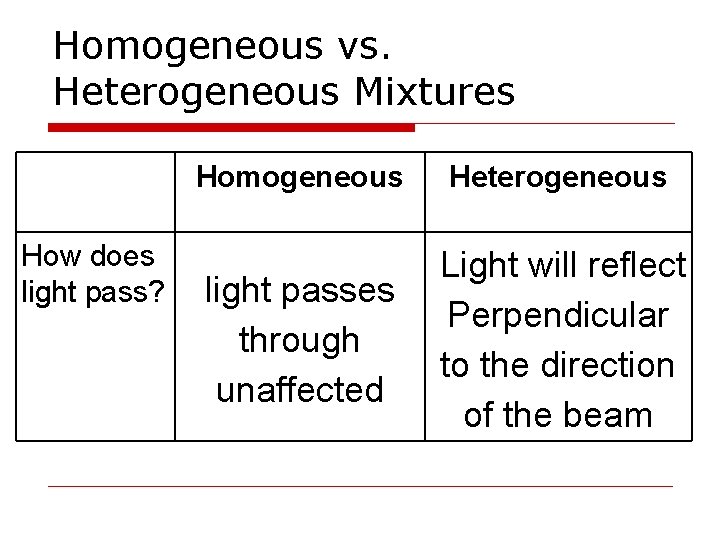
Homogeneous vs. Heterogeneous Mixtures How does light pass? Homogeneous Heterogeneous light passes through unaffected Light will reflect Perpendicular to the direction of the beam
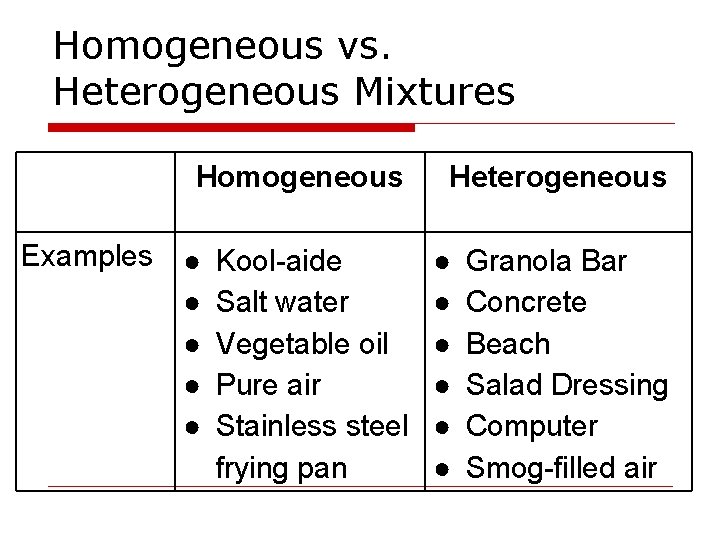
Homogeneous vs. Heterogeneous Mixtures Homogeneous Examples ● ● ● Kool-aide Salt water Vegetable oil Pure air Stainless steel frying pan Heterogeneous ● ● ● Granola Bar Concrete Beach Salad Dressing Computer Smog-filled air

The Tyndall Effect Light can be used to distinguish between solutions and what appears to be a solution • cannot be used to distinguish between a solution and a pure liquid
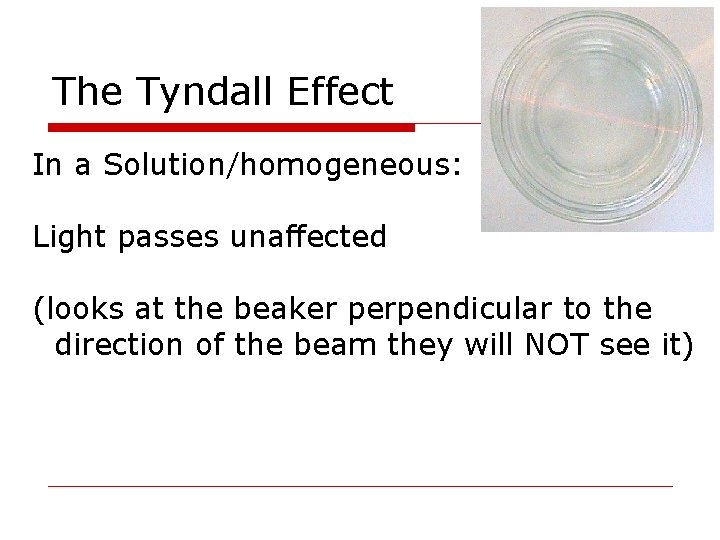
The Tyndall Effect In a Solution/homogeneous: Light passes unaffected (looks at the beaker perpendicular to the direction of the beam they will NOT see it)
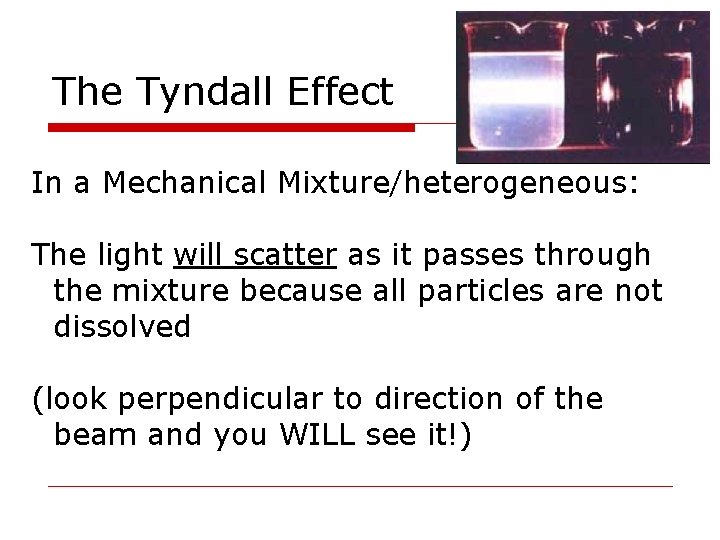
The Tyndall Effect In a Mechanical Mixture/heterogeneous: The light will scatter as it passes through the mixture because all particles are not dissolved (look perpendicular to direction of the beam and you WILL see it!)
Partner Activity List the various homogeneous and heterogeneous mixtures in your home. Homogeneous Heterogeneous

Mini-Lab: Compare Milk, OJ and Soda Pure or Mixture? Pg. 238 -239 Are they solutions or mixtures? Homogeneous or Heterogeneous? Did the extra magnification change you mind?

Chapter Review Questions: Page. 250 -251: 1, 2, 4, 6 & 8
- Slides: 29