Grade 11 Chemistry Unit 4 Chemical Reactions Text








- Slides: 13

Grade 11 Chemistry Unit 4 – Chemical Reactions (Text Ch. 3 p. 106 - 146)

Chemical Reactions The essence of chemical reactions is the making of a new substance, through a chemical process. Mass is conserved in a chemical reaction. The mass of the reactants, the amounts of the reactants, must equal the mass or amounts of products. n (Law of Conservation of Mass)

Chemical Reactions This means that new bonds are formed by the rearranging of old bonds to make a new substance If a new substance does not form, a chemical reaction does not take place. We also need to see an indicator of a chemical reaction.

Chemical Reactions All chemical reactions are based upon energy. If there is enough energy, the reaction goes; if not, the reaction does not occur. n n 95% of all reactions will go to completion (REACTANTS to PRODUCTS) These reactions are considered SPONTANEOUS REACTIONS

Chemical Reactions Where does the energy come from? n Internal Bonds between reactants When bonds break, E is given off If there is enough E, the reaction takes place n External Outside source, like a burner, sun (uv light), etc.

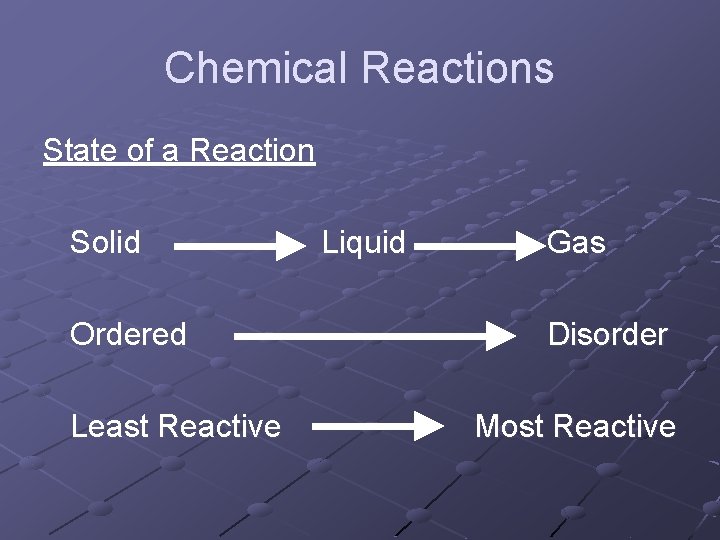
Chemical Reactions What state will cause the greatest reaction? n When something is unstable it will react to create a stable substance Disorder n Order This change of disorder to order arrangement is called Entropy

Chemical Reactions State of a Reaction Solid Ordered Least Reactive Liquid Gas Disorder Most Reactive

Chemical Reactions Due to the fact gases are difficult to control, we prefer to use liquids Since you cannot really “liquefy” solids, without a lot of energy, we dissolve them in water to form a more reactive solution, as the particles are spread out, and the reaction will occur faster.

Chemical Reactions The Mechanism for Chemical Change n Read p. 109 – 110. Take notes on Kinetic Molecular Theory, how the Kinetic Molecular Theory relates to the Collision (Reaction) Theory and chemical reactions occurring.

Chemical Reactions The chemical reaction is the recipe. The chemical equation shows the ingredients; it shows the formulas of the reactants and the resulting products.

Chemical Reactions Rules of Balancing Equations n n n Separate the products and reactants Count up atoms or polyatomic ions on both sides of the equation Order of balancing Balance metals Balance non-metals Balance Hydrogen and then Oxygen

Chemical Reactions Types of Chemical Reactions n There are 5 types of reactions: Synthesis Decomposition Single Displacement Double Displacement Combustion Read the pages 114 – 139. Define each of the above reactions, give an example for each.

Chemical Reactions Determining whether a chemical reaction takes place n There are several diagnostic tests that can be done. Using the lab results below, and some internet resources: n n How can we determine whether a chemical reaction has taken place? What are the basic diagnostic tests used?