Grade 10 MATTER AND MATERIALS UNIT 1 MACROSCOPIC







- Slides: 26

Grade 10 MATTER AND MATERIALS UNIT 1: MACROSCOPIC PROPERTIES

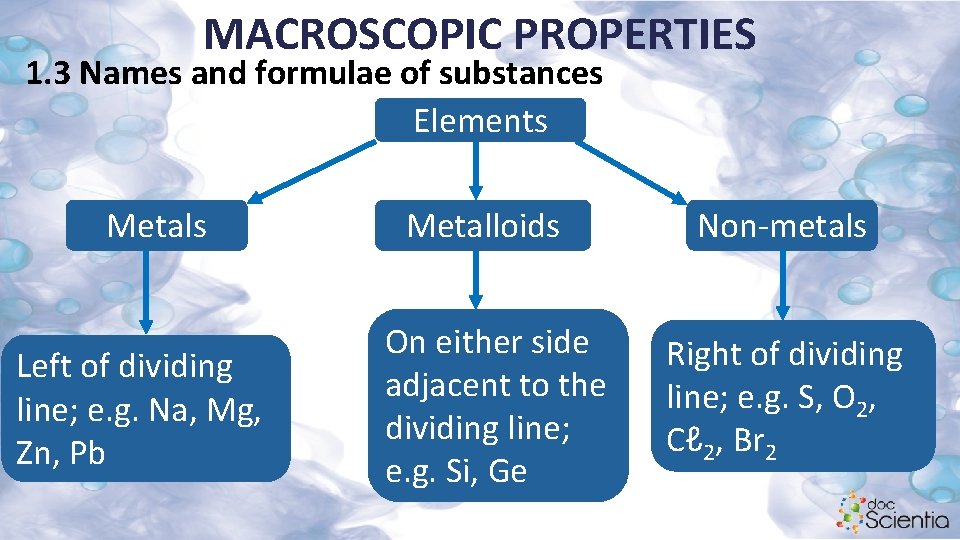
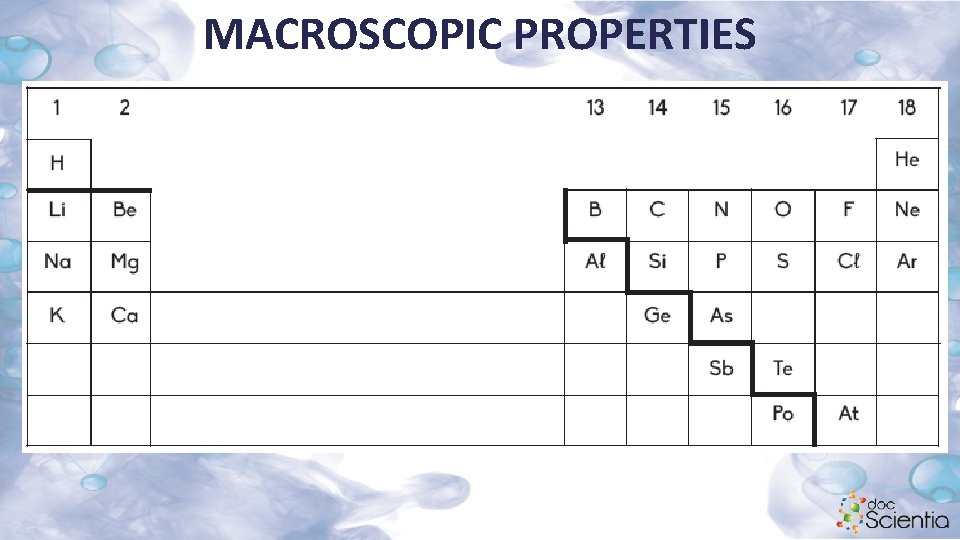
MACROSCOPIC PROPERTIES 1. 3 Names and formulae of substances Elements Metals Left of dividing line; e. g. Na, Mg, Zn, Pb Metalloids On either side adjacent to the dividing line; e. g. Si, Ge Non-metals Right of dividing line; e. g. S, O 2, Cℓ 2, Br 2

MACROSCOPIC PROPERTIES

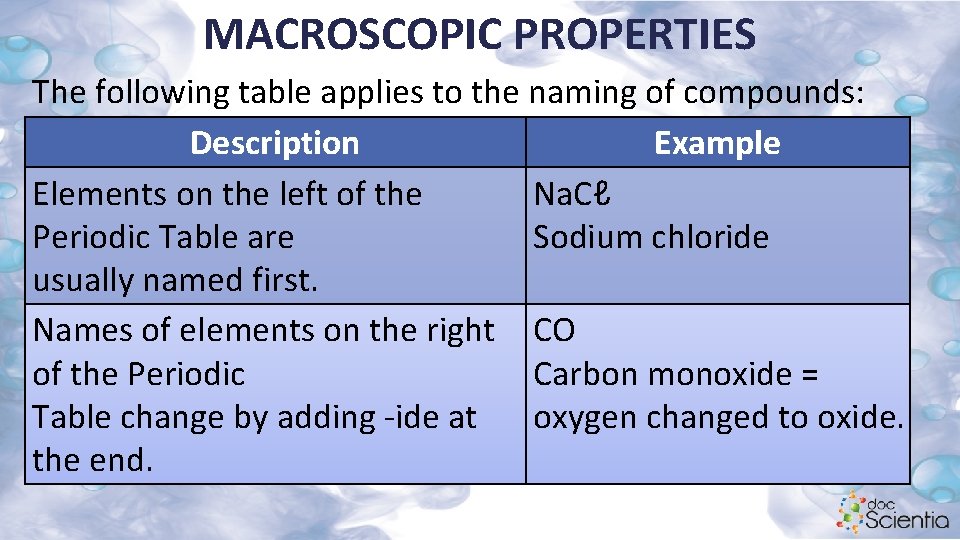
MACROSCOPIC PROPERTIES The following table applies to the naming of compounds: Description Example Elements on the left of the Na. Cℓ Periodic Table are Sodium chloride usually named first. Names of elements on the right CO of the Periodic Carbon monoxide = Table change by adding -ide at oxygen changed to oxide. the end.

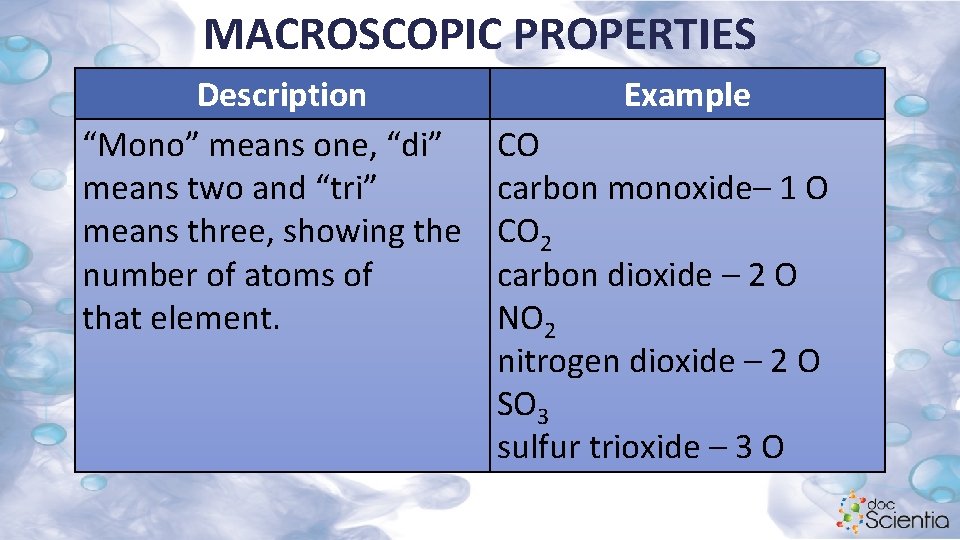
MACROSCOPIC PROPERTIES Description “Mono” means one, “di” means two and “tri” means three, showing the number of atoms of that element. Example CO carbon monoxide– 1 O CO 2 carbon dioxide – 2 O NO 2 nitrogen dioxide – 2 O SO 3 sulfur trioxide – 3 O

MACROSCOPIC PROPERTIES Description Example Some substances have H 2 O common names, too. hydrogen oxide = water HCℓ hydrogen chloride = hydrochloric acid = swimming pool acid

MACROSCOPIC PROPERTIES Description Polyatomic ions, which always consist of the same ratio of elements (law of constant composition). Example Na 2 SO 4 sodium sulfate Ca. SO 4 calcium sulfate Mg. SO 4 magnesium sulfate

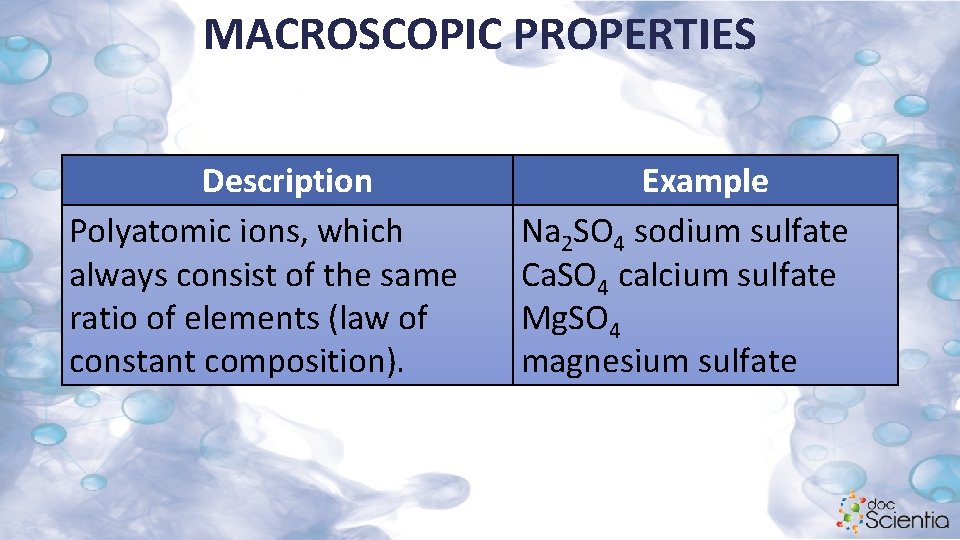
MACROSCOPIC PROPERTIES To write a formula you need to take into account the constituents as well as the ratio in which they are combined. • Water consists of hydrogen and oxygen in the ratio 2: 1. Therefore the formula is H 2 O. Two hydrogen atoms and one oxygen atom:

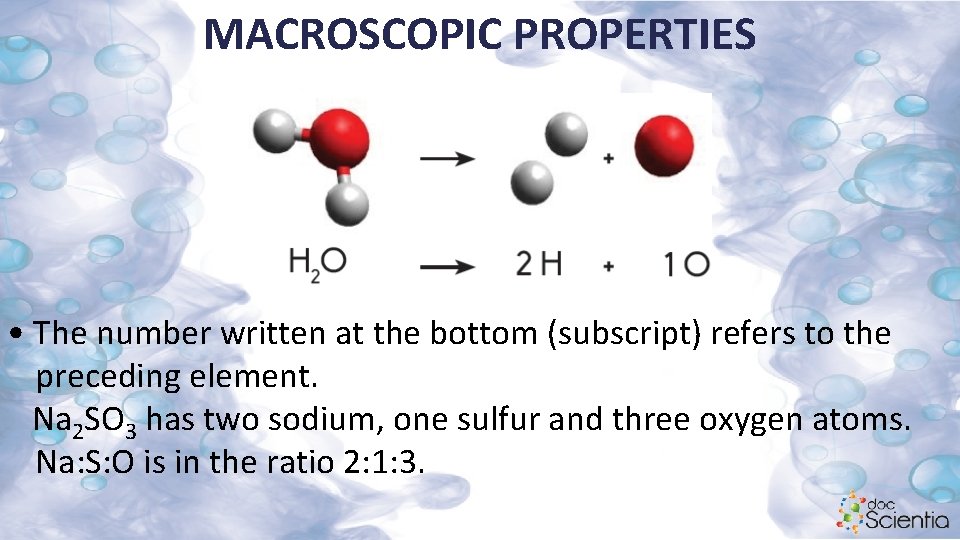
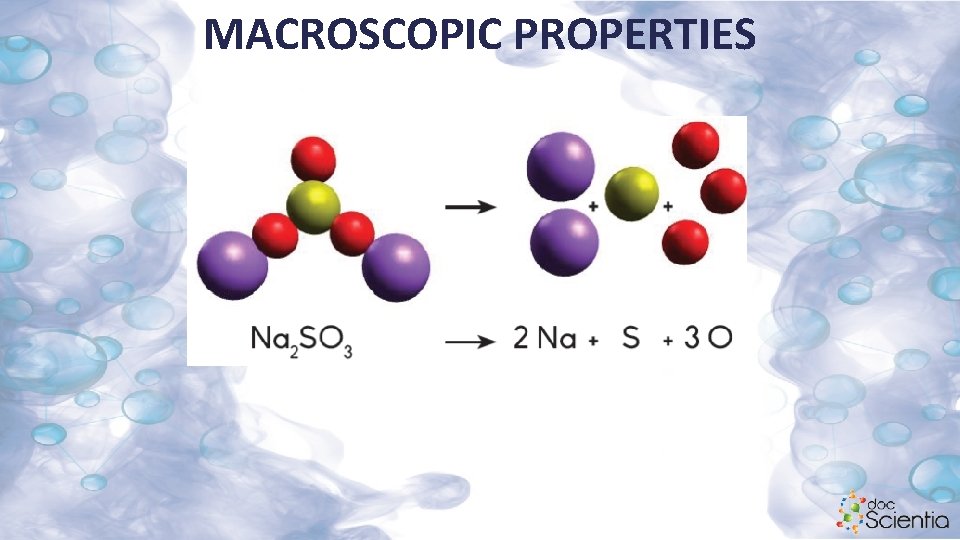
MACROSCOPIC PROPERTIES • The number written at the bottom (subscript) refers to the preceding element. Na 2 SO 3 has two sodium, one sulfur and three oxygen atoms. Na: S: O is in the ratio 2: 1: 3.

MACROSCOPIC PROPERTIES

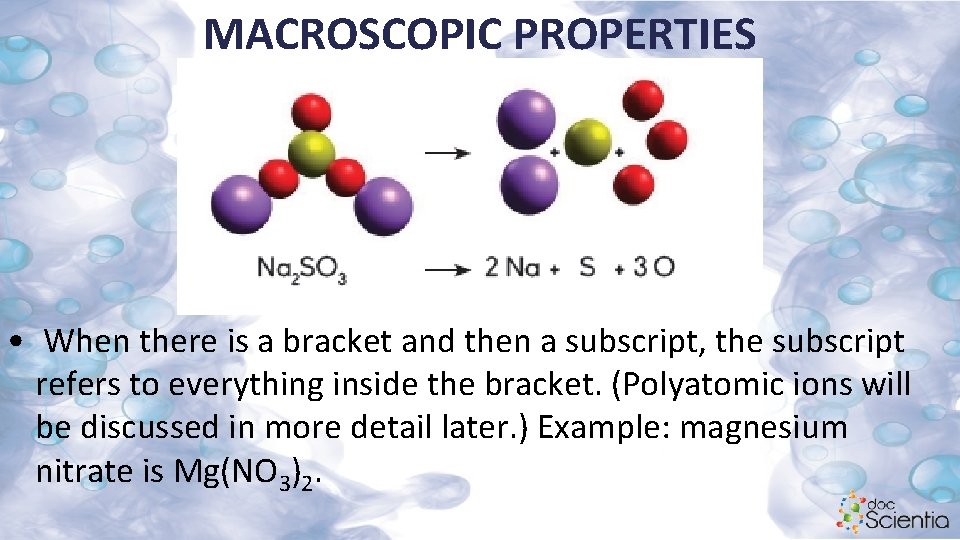
MACROSCOPIC PROPERTIES • When there is a bracket and then a subscript, the subscript refers to everything inside the bracket. (Polyatomic ions will be discussed in more detail later. ) Example: magnesium nitrate is Mg(NO 3)2.

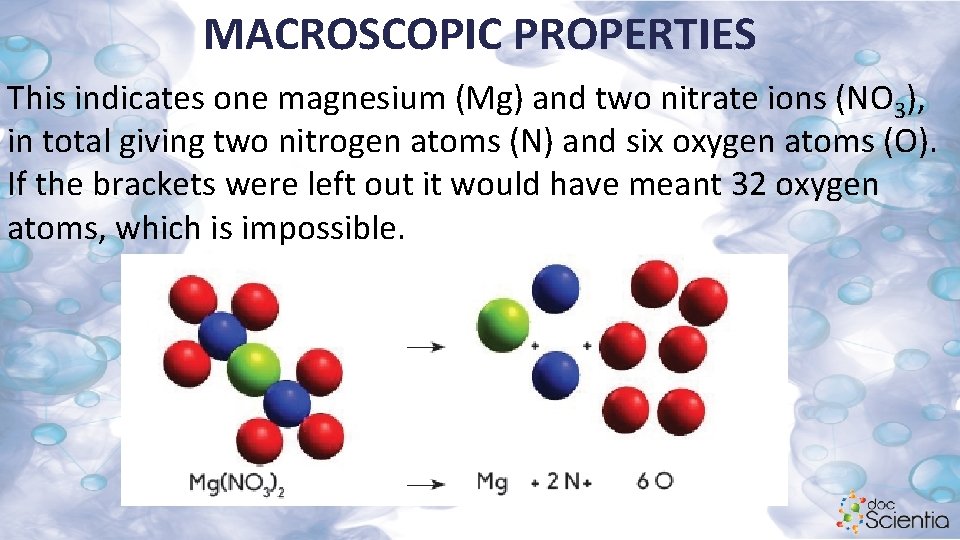
MACROSCOPIC PROPERTIES This indicates one magnesium (Mg) and two nitrate ions (NO 3), in total giving two nitrogen atoms (N) and six oxygen atoms (O). If the brackets were left out it would have meant 32 oxygen atoms, which is impossible.

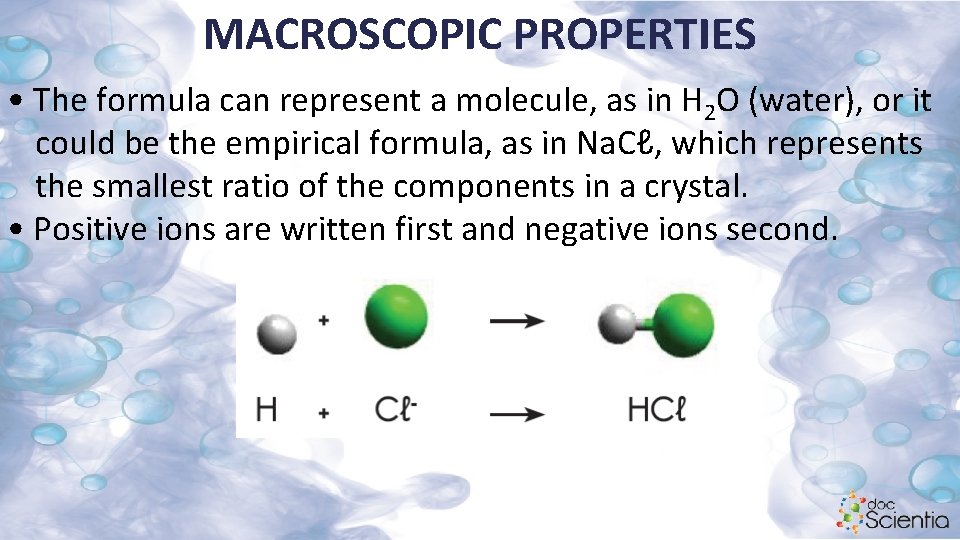
MACROSCOPIC PROPERTIES • The formula can represent a molecule, as in H 2 O (water), or it could be the empirical formula, as in Na. Cℓ, which represents the smallest ratio of the components in a crystal. • Positive ions are written first and negative ions second.

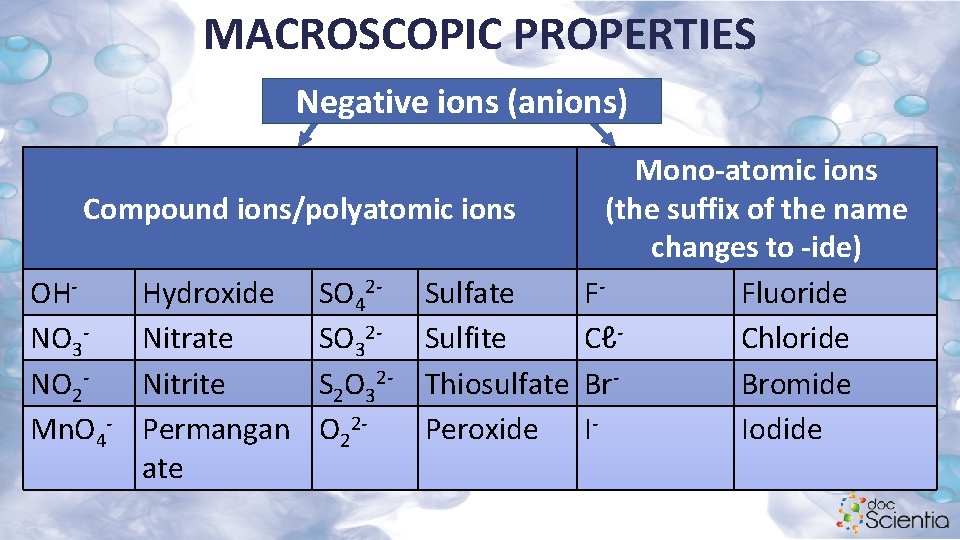
MACROSCOPIC PROPERTIES Negative ions (anions) Mono-atomic ions Compound ions/polyatomic ions (the suffix of the name changes to -ide) OHHydroxide SO 42 - Sulfate FFluoride NO 3 Nitrate SO 32 - Sulfite CℓChloride NO 2 Nitrite S 2 O 32 - Thiosulfate Br. Bromide Mn. O 4 - Permangan O 22 Peroxide IIodide ate

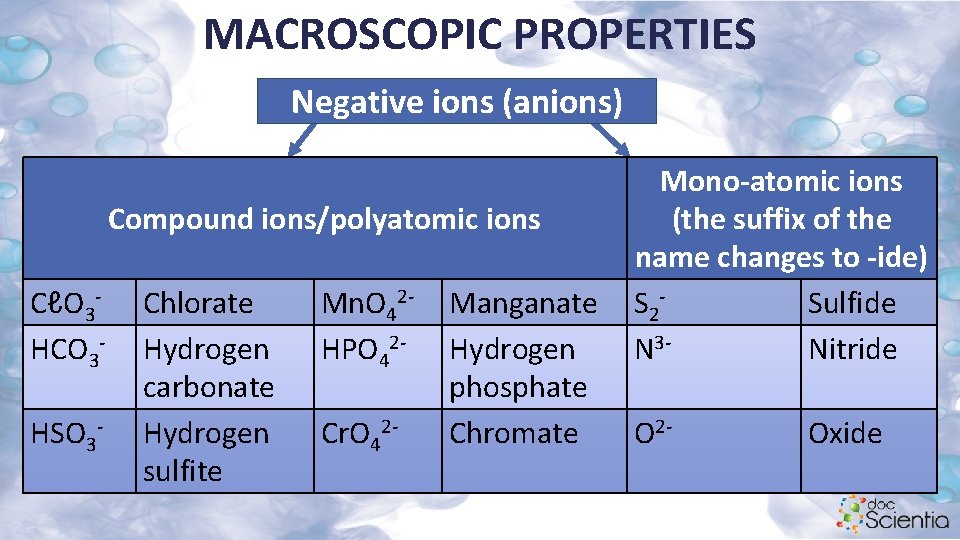
MACROSCOPIC PROPERTIES Negative ions (anions) Compound ions/polyatomic ions CℓO 3 HCO 3 HSO 3 - Chlorate Hydrogen carbonate Hydrogen sulfite Mn. O 42 HPO 42 Cr. O 42 - Manganate Hydrogen phosphate Chromate Mono-atomic ions (the suffix of the name changes to -ide) S 2 Sulfide N 3 Nitride O 2 - Oxide

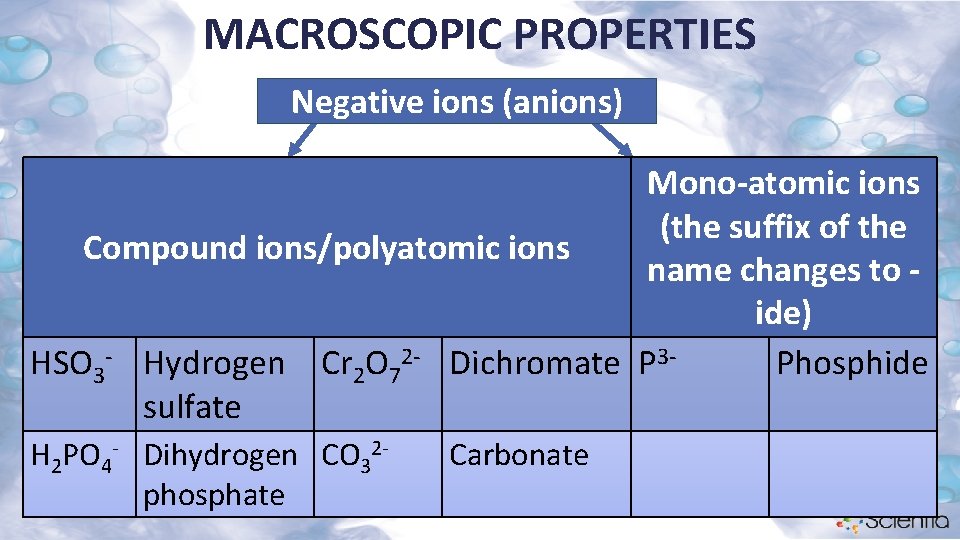
MACROSCOPIC PROPERTIES Negative ions (anions) Mono-atomic ions (the suffix of the Compound ions/polyatomic ions name changes to ide) HSO 3 - Hydrogen Cr 2 O 72 - Dichromate P 3 Phosphide sulfate H 2 PO 4 - Dihydrogen CO 32 phosphate Carbonate

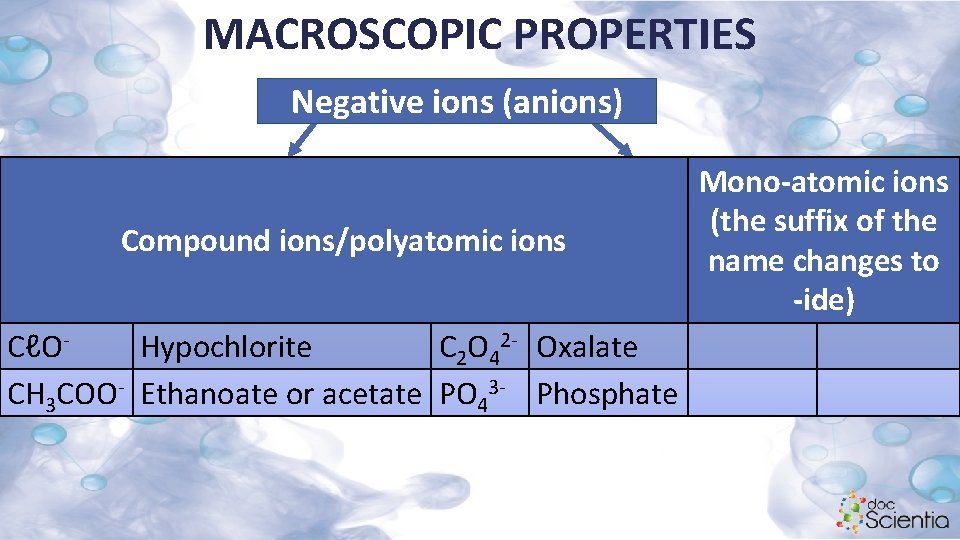
MACROSCOPIC PROPERTIES Negative ions (anions) Compound ions/polyatomic ions CℓOHypochlorite C 2 O 42 - Oxalate CH 3 COO- Ethanoate or acetate PO 43 - Phosphate Mono-atomic ions (the suffix of the name changes to -ide)

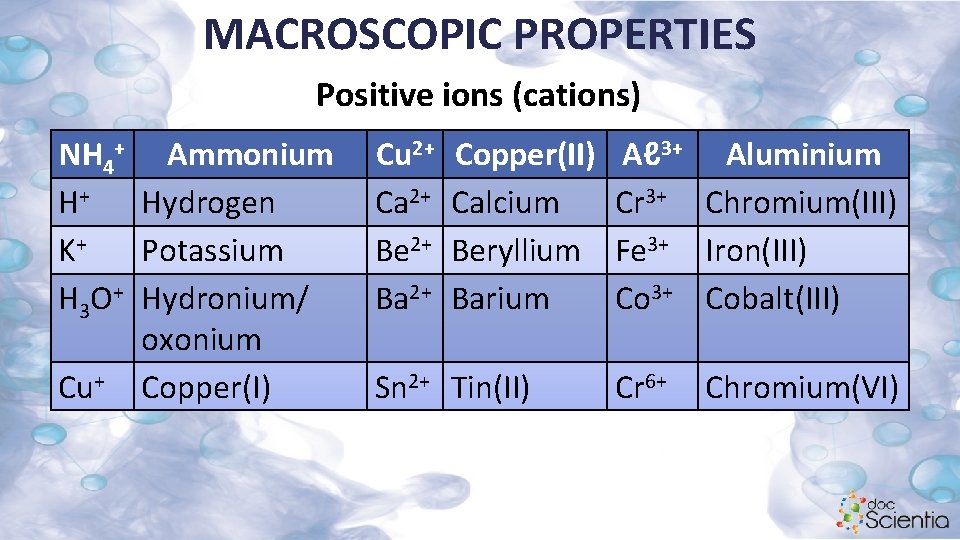
MACROSCOPIC PROPERTIES Positive ions (cations) NH 4+ Ammonium H+ Hydrogen K+ Potassium H 3 O+ Hydronium/ oxonium Cu+ Copper(I) Cu 2+ Ca 2+ Be 2+ Ba 2+ Copper(II) Calcium Beryllium Barium Sn 2+ Tin(II) Aℓ 3+ Aluminium Cr 3+ Chromium(III) Fe 3+ Iron(III) Co 3+ Cobalt(III) Cr 6+ Chromium(VI)

MACROSCOPIC PROPERTIES Ag+ Silver Mg 2+ Lithium Cr 2+ Mn 2+ Fe 2+ Co 2+ Ni 2+ Magnesium Mn 2+ Manganese(VII) Chromium(II) Manganese(II) Iron(II) Cobalt(II) Nickel(II)

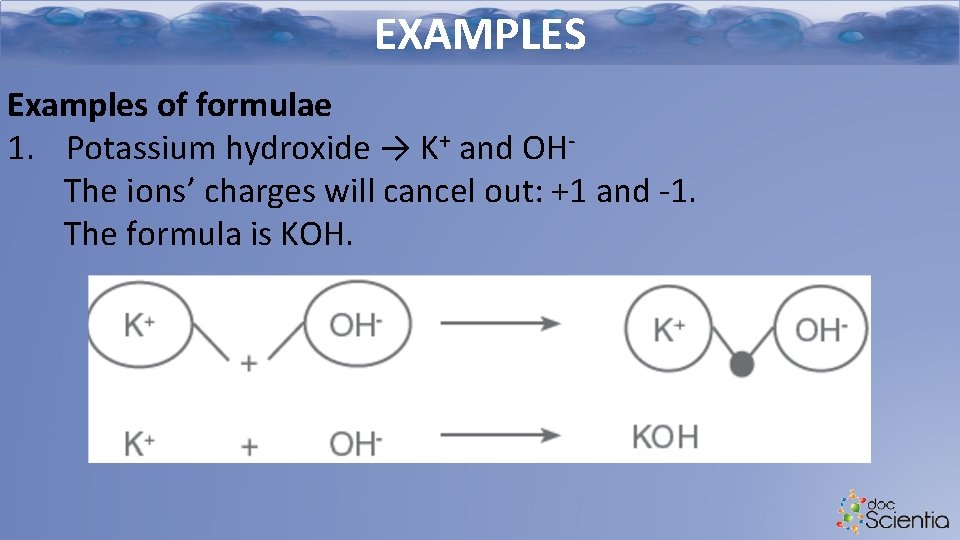
EXAMPLES Examples of formulae 1. Potassium hydroxide → K+ and OHThe ions’ charges will cancel out: +1 and -1. The formula is KOH.

EXAMPLES 2. Potassium sulfate → K+ and SO 42 Two positive ions are needed to cancel out the two negative charges. 2 × K+ and SO 42 -: +2 and -2. The formula is K 2 SO 4.

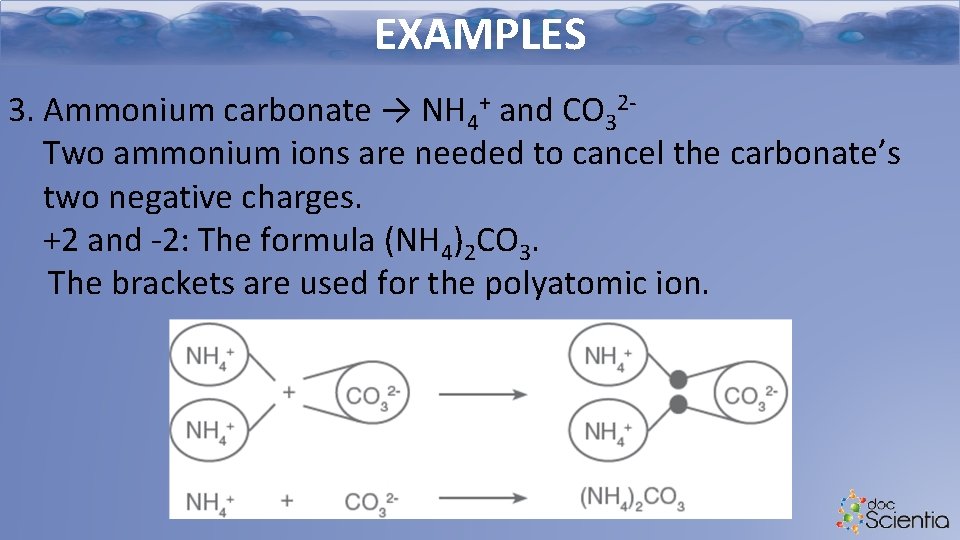
EXAMPLES 3. Ammonium carbonate → NH 4+ and CO 32 Two ammonium ions are needed to cancel the carbonate’s two negative charges. +2 and -2: The formula (NH 4)2 CO 3. The brackets are used for the polyatomic ion.

MACROSCOPIC PROPERTIES Chemical and common name of compounds Formula Chemical name H 2 O Hydrogen oxide NH 3 Hydrogen nitride HCℓ Hydrogen chloride H 2 SO 3 H 2 SO 4 HNO 3 H 2 CO 3 Hydrogen sulfite Hydrogen sulfate Hydrogen nitrate Hydrogen carbonate Common name Water Ammonia Hydrochloric acid/swimming pool acid Sulfuric acid/battery acid Nitric acid Carbonic acid/soda water

Formula H 3 PO 4 CH 3 COOH (COOH)2 Na. Cℓ Na. OH Na. HCO 3 Na 2 CO 3 Na. NO 3 MACROSCOPIC PROPERTIES Chemical name Hydrogen phosphate Ethanoic acid Ethanedioic acid Sodium chloride Sodium hydroxide Sodium hydrogen carbonate Sodium nitrate Common name Phosphoric acid Vinegar Oxalic acid Table salt Caustic soda Baking soda (Bicarbonate of soda) Washing soda Chile saltpetre

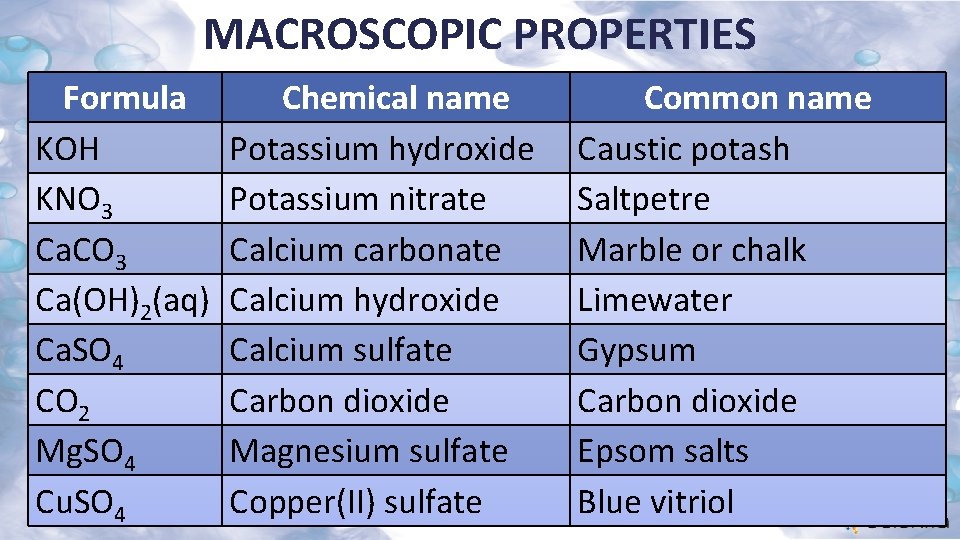
MACROSCOPIC PROPERTIES Formula KOH KNO 3 Ca. CO 3 Ca(OH)2(aq) Ca. SO 4 CO 2 Mg. SO 4 Cu. SO 4 Chemical name Potassium hydroxide Potassium nitrate Calcium carbonate Calcium hydroxide Calcium sulfate Carbon dioxide Magnesium sulfate Copper(II) sulfate Common name Caustic potash Saltpetre Marble or chalk Limewater Gypsum Carbon dioxide Epsom salts Blue vitriol

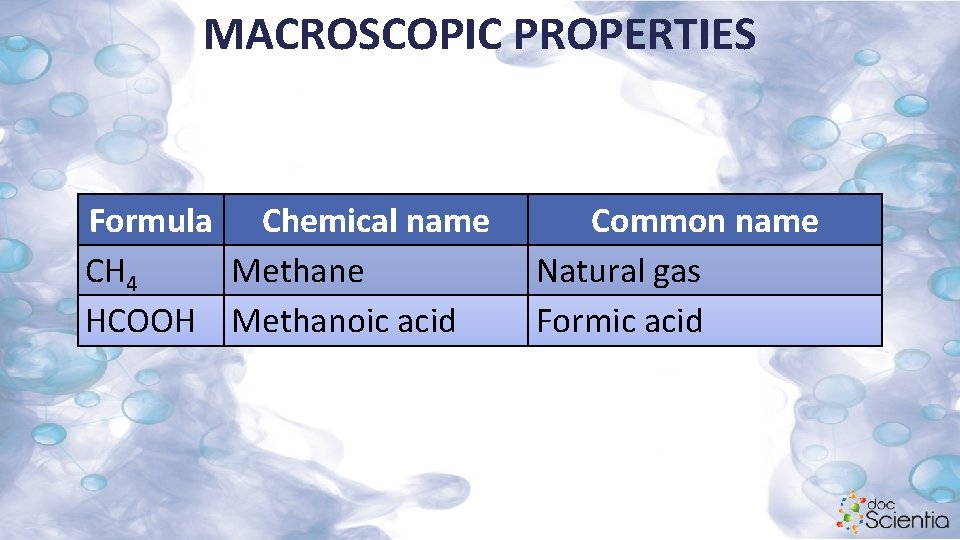
MACROSCOPIC PROPERTIES Formula Chemical name CH 4 Methane HCOOH Methanoic acid Common name Natural gas Formic acid