Good use in conjunction with flow chart Matter














- Slides: 26

¢ Good use in conjunction with flow chart


Matter describing matter

Matter ¢ matter: anything that has mass and takes up space

Properties of Matter property: characteristic ¢ Types of Properties ¢ 1. physical properties 2. chemical properties physical and chemical properties are used to identify, describe, and classify matter

Physical Properties ¢ Observed without changing the substance into something else ¢ Examples l Color l Density l Shape l Boiling Point l Odor l Solubility l Hardness l Malleability l Ductility

Chemical Properties ¢ Observed only when substance is changed and interacts with another substance ¢ Examples l flammability: able to burn l rusting: combining with oxygen to form rust

Physical and Chemical Properties ¢ ¢ What is an example of a physical property of a candle? What is an example of a chemical property of a candle?

3 States of Matter

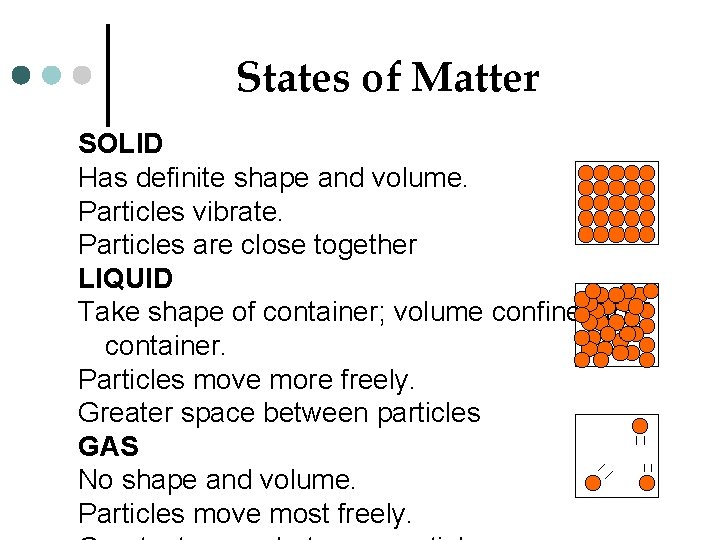
States of Matter SOLID Has definite shape and volume. Particles vibrate. Particles are close together LIQUID Take shape of container; volume confined to container. Particles move more freely. Greater space between particles GAS No shape and volume. Particles move most freely.

Kinds of Matter ¢ Fundamental kinds of matter interact to form everything around us l Elements l Compounds l Mixtures

Pure Substance ¢ A substance made up of only 1 particle throughout ¢ Pure substances can be elements, compounds, molecules, atoms

¢ A pure substance that cannot be broken down into other substances chemically or physically Made up of only 1 type of atom ¢ examples ¢ Elements l l l sodium oxygen carbon aluminum sulfur

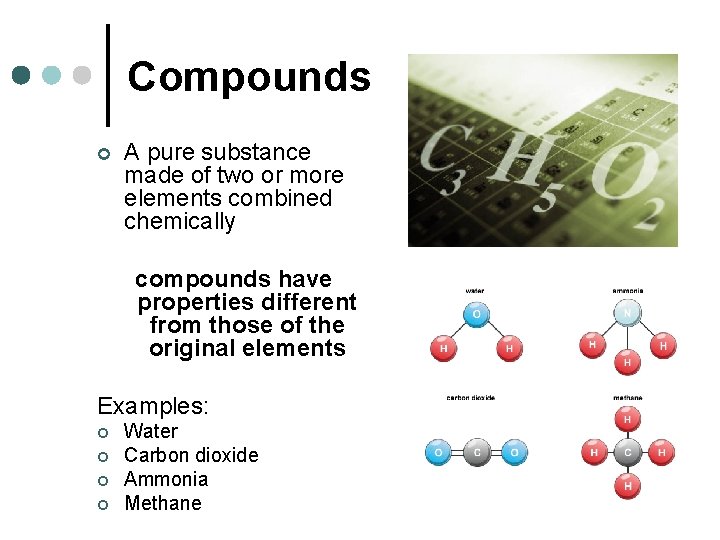
Compounds ¢ A pure substance made of two or more elements combined chemically compounds have properties different from those of the original elements Examples: ¢ ¢ Water Carbon dioxide Ammonia Methane

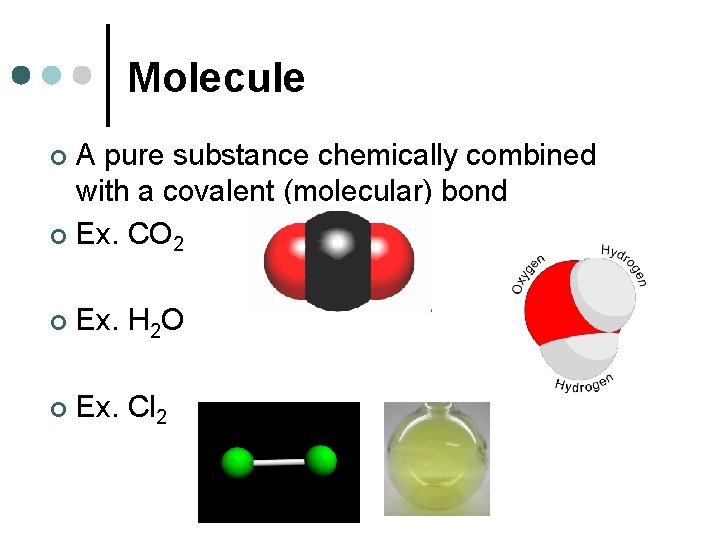
Molecule A pure substance chemically combined with a covalent (molecular) bond ¢ Ex. CO 2 ¢ ¢ Ex. H 2 O ¢ Ex. Cl 2

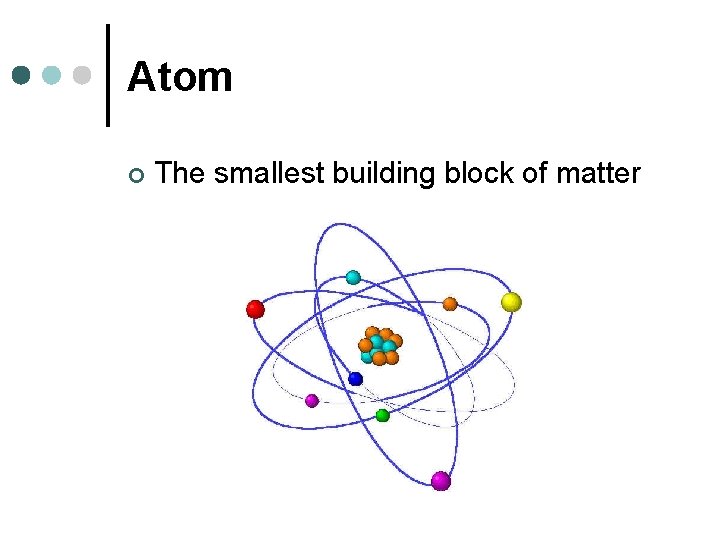
Atom ¢ The smallest building block of matter

Mixtures ¢ ¢ combination of two or more substances that are not chemically combined examples l l l salad frosted cake kool-aid

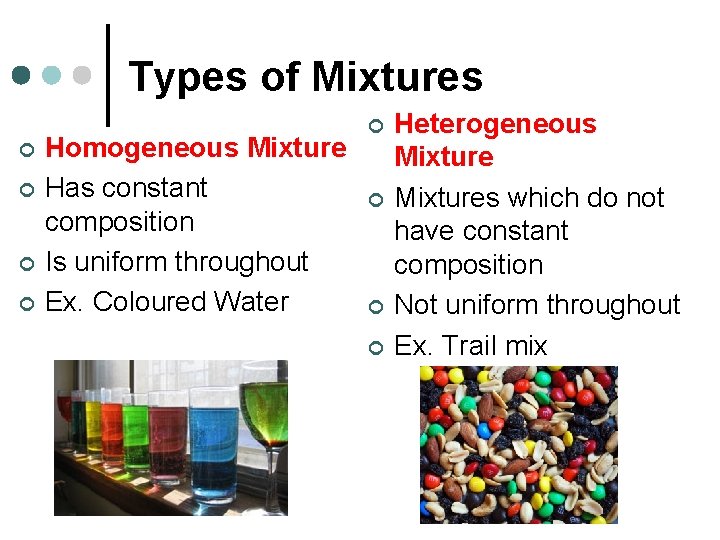
Types of Mixtures ¢ ¢ Homogeneous Mixture Has constant composition Is uniform throughout Ex. Coloured Water ¢ ¢ Heterogeneous Mixtures which do not have constant composition Not uniform throughout Ex. Trail mix

Changes in matter ¢ two kinds physical changes l chemical changes l energy is used anytime a change in matter occurs

physical change ¢ ¢ Alters form or appearance of material, but does not change material into brand new substance examples l l l ¢ chopping wood bending wire molding clay phase changes

chemical change ¢ ¢ produces new substances examples l l wood burning sour milk

Chemical Change ¢ ¢ the substance is altered chemically and displays different physical and chemical properties after the change. new substance(s) are formed through reorganization of atoms. A chemical change is irreversible Ex. Magnesium wire burns producing white ash of Mg. O ¢ Ex. Zinc is combined with Hydrochloric Acid ¢ Ex. Food metabolizes in the body

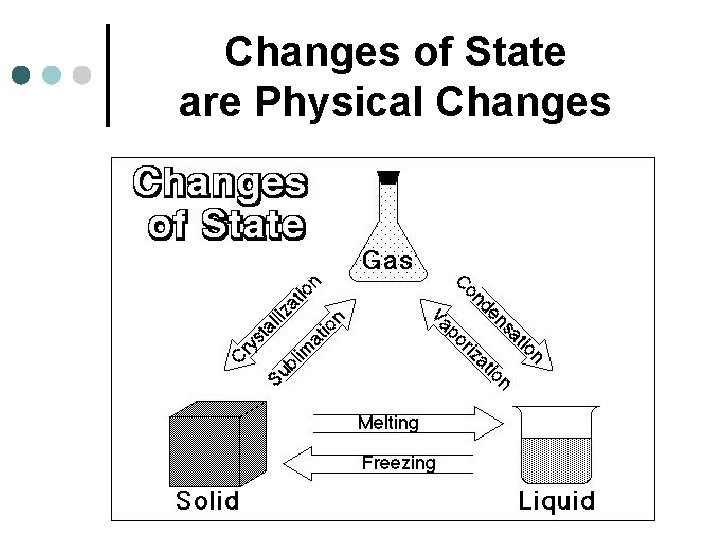
Changes of State are Physical Changes

¢ ¢ ¢ Crystallization/Deposition: the change in state from a gas to solid Sublimation: the change in state from solid to gas Condensing: the change in state from gas to liquid Vaporization: the change in state from liquid to gas Melting: the change in state from solid to liquid Freezing: the change in state from liquid to solid

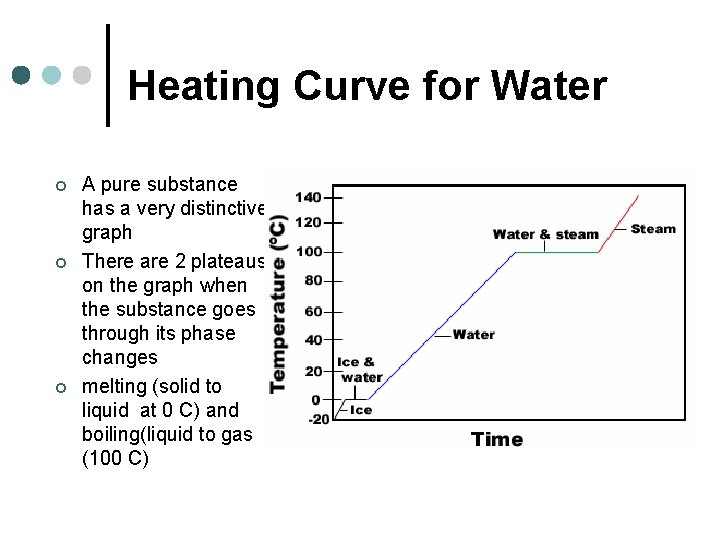
Heating Curve for Water ¢ ¢ ¢ A pure substance has a very distinctive graph There are 2 plateaus on the graph when the substance goes through its phase changes melting (solid to liquid at 0 C) and boiling(liquid to gas (100 C)

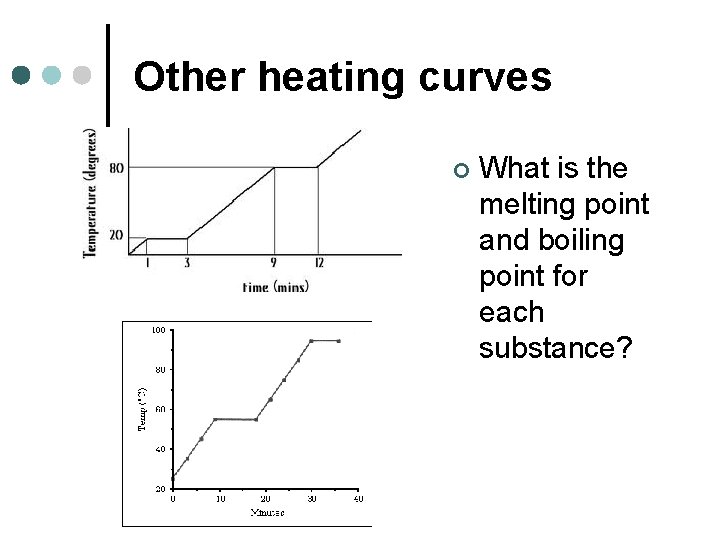
Other heating curves ¢ What is the melting point and boiling point for each substance?