Good Manufacturing Practices Dr Chander Aurora Regulatory Lifecycle






















- Slides: 29

Good Manufacturing Practices Dr. Chander Aurora

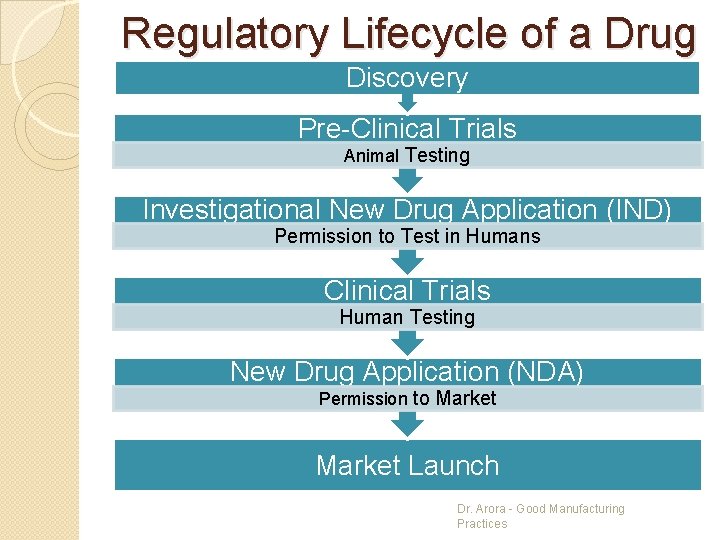
Regulatory Lifecycle of a Drug Discovery Pre-Clinical Trials Animal Testing Investigational New Drug Application (IND) Permission to Test in Humans Clinical Trials Human Testing New Drug Application (NDA) Permission to Market Launch Dr. Arora - Good Manufacturing Practices

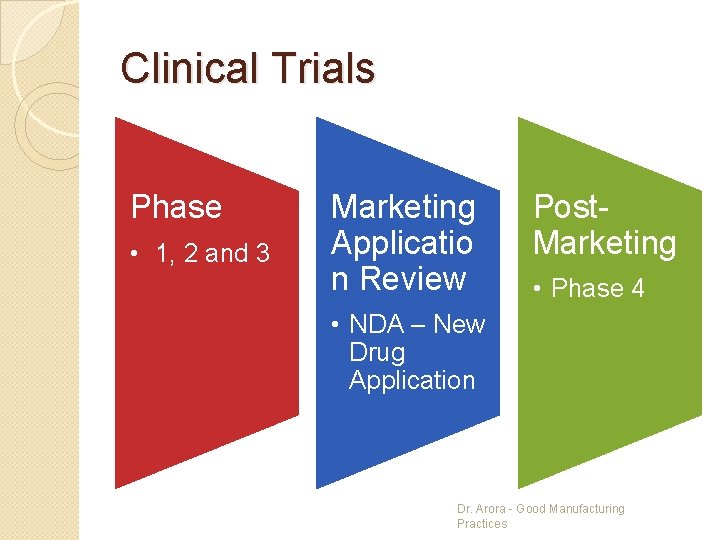
Clinical Trials Phase • 1, 2 and 3 Marketing Applicatio n Review Post. Marketing • Phase 4 • NDA – New Drug Application Dr. Arora - Good Manufacturing Practices

Manufacturing is �Even more disciplined activity. . . �GMP takes priority �QC becomes an important driver! Dr. Arora - Good Manufacturing Practices

International Harmonization �c. GMPs exist in all major markets ◦ Europe ◦ Japan ◦ US �International Conference on Harmonization has aligned c. GMPs between major markets ◦ Interpretation can still be issue ◦ Emphasis different from market to market Dr. Arora - Good Manufacturing Practices

Objectives l To understand where the regulations are coming from? l Who enforces them? l Why do we need to comply? Dr. Arora - Good Manufacturing Practices

Objectives continued l To understand the: ◦ Fundamentals ◦ Benefits ◦ Key parts of GMP - Good Manufacturing Practices Dr. Arora - Good Manufacturing Practices

Compliance �Written into Federal Register – mandatory regulations �GLPs (Good Laboratory Practices) ◦ Pre-clinical, EPA �GCPs (Good Clinical Practices) ◦ Clinical studies �GMPs (Good Manufacturing Practices) ◦ Production and distribution of drugs and devices for human use Dr. Arora - Good Manufacturing Practices

GMP for what? l The acronym "GMP" (Good Manufacturing Practice) is used internationally to describe: ◦ A set of principles and procedures which, when followed by manufacturers of therapeutic goods �Helps ensure that the products manufactured will have the required quality. Dr. Arora - Good Manufacturing Practices

GMP – Why? l Regulations & Guidelines ◦ History & Current l GMP works in conjunction with ◦ Quality Control, QC ◦ Quality Assurance, QA Dr. Arora - Good Manufacturing Practices

GMP or c. GMP l Current -c l Good Manufacturing Practices l Comes from Food Drug and Cosmetic Act l Rules set up by FDA ◦ Food and Drug Administration ◦ Drug Manufacturers need to follow in order to ensure that a safe and effective product is manufactured for the consumers Dr. Arora - Good Manufacturing Practices

GMPs Importance l Government l Ensures Quality Product l Reduces Rejects & Recalls l Maintains l Satisfied Requirement Manufacturing Consistency Customers l Company Image & Reputation Dr. Arora - Good Manufacturing Practices

Enforcement l FDA (Food & Drug Administration) ◦ Interprets and Enforces the law l FDA is an Agency under ◦ Department of Health & Human Services �(DHHS) l FDA has EIGHT branches Dr. Arora - Good Manufacturing Practices

FDA Organization and Structure Commission er Office of AIDS and Special Health Issues Office of External Affairs Office of Consumer Affairs Office of Health Affairs Office of Legislative Affairs Office of Public Affairs Office of Women’s Health Office of Management and Systems Office of Operations Office of Information Resources and Management Office of Planning and Evaluation Center for Biologics Evaluation and Research Center for Devices and Radiological Health Center for Drug Evaluation and Research Center for Food Safety and Applied Nutrition Center for Veterinary Medicine National Center for Toxicological Research Office of Orphan Products Development Office of Regulatory Affairs Regulations Policy and Management Staff Office of Policy Development and Coordination Staff Policy Research Staff International Policy Staff Dr. Arora - Good Manufacturing Practices

GMP Regulations l. Current Good Manufacturing Practices for Pharmaceuticals (c. GMP) ◦ Proposed by FDA February 13, 1976 ◦ Published in final form in CFR September 29, 1978 ◦ Effective March 28, 1979 Dr. Arora - Good Manufacturing Practices

c. GMP Requirements l c. GMP and the Act assures ◦ The manufactured Drug meets and possess the characteristics for which it has been approved: �Safety �Identity �Strength �Quality �Purity Dr. Arora - Good Manufacturing Practices

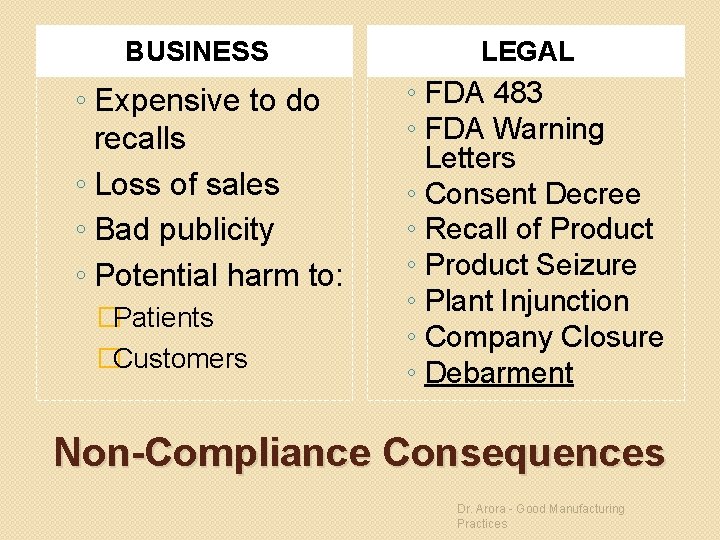
BUSINESS ◦ Expensive to do recalls ◦ Loss of sales ◦ Bad publicity ◦ Potential harm to: �Patients �Customers LEGAL ◦ FDA 483 ◦ FDA Warning Letters ◦ Consent Decree ◦ Recall of Product ◦ Product Seizure ◦ Plant Injunction ◦ Company Closure ◦ Debarment Non-Compliance Consequences Dr. Arora - Good Manufacturing Practices

Validation l Definition ◦ Documented evidence that provides a high degree of assurance that a specific process, facility, or support system will consistently produce a product meeting its predetermined specifications and quality attributes. ◦ Validation helps assure that: �Products are pure, safe and effective Dr. Arora - Good Manufacturing Practices

Benefits of c. GMP l c. GMPs outlines a Quality System ◦ Reduces / Prevents Errors ◦ Ensures that products are safe for humans use ◦ Prevents & Controls Contamination / Cross contamination ◦ Minimizes Potency variation of drugs �Prevents side effects ◦ Ensures reproducible physiological activity ◦ Prevents mislabeling and adulteration Dr. Arora - Good Manufacturing Practices

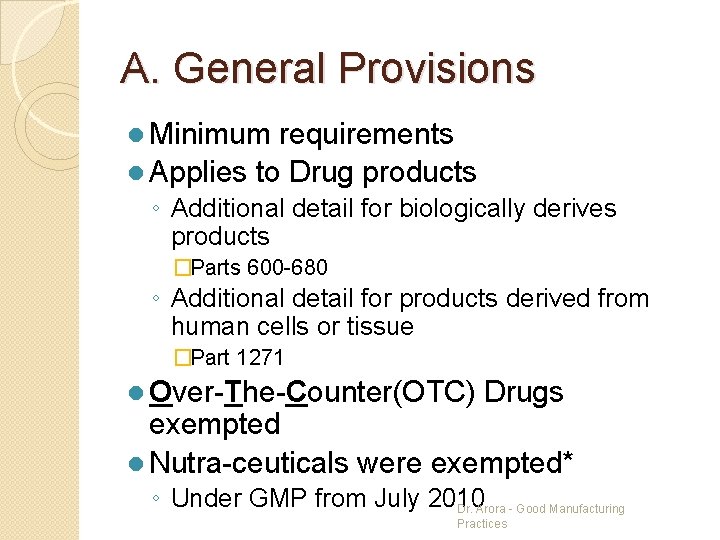
A. General Provisions l Minimum requirements l Applies to Drug products ◦ Additional detail for biologically derives products �Parts 600 -680 ◦ Additional detail for products derived from human cells or tissue �Part 1271 l Over-The-Counter(OTC) Drugs exempted l Nutra-ceuticals were exempted* ◦ Under GMP from July 2010 Dr. Arora - Good Manufacturing Practices

Standard Operating Procedures � Good ◦ ◦ SOPs contain the following: Purpose or scope Materials Procedure �Must be written in proper order �Must include sufficient detail for operator to follow �Should include “checkers” for calculations �Must include initials and signature of operator �Must have Supervisors review and sign off Should be reviewed and updated as needed Dr. Arora - Good Manufacturing Practices

Batch Records l FDA Requirement l c. GMP requirement l QSR requirement l ISO requirement l Failing to do so can get you into a heap of trouble! l Used to ensure consistent product Dr. Arora - Good Manufacturing Practices

Batch Record: Record Keeping l. Record keeping requirements ◦ Black ink ◦ No white out ◦ Single line, Date, Initial ◦ No blanks �N/A any lines which do not apply ◦ Legible Dr. Arora - Good Manufacturing Practices

Who is responsible for GMP? Everyon e Dr. Arora - Good Manufacturing Practices

TRUST Manufacturer Doctor & Pharmacist Patient Dr. Arora - Good Manufacturing Practices

What does FDA do? l Approve products ◦ Safety ◦ Effectiveness ◦ Risk/Benefit determination l Monitor drug performance l Monitor investigational studies l Inspect manufacturers l Inform physicians and consumers Dr. Arora - Good Manufacturing Practices

FDA and Blood Donations l Do they oversee Blood Donations? l. YES! l What testing or procedures do they require? ◦ Donor screening: medical history ◦ Blood testing: for HIV, Hep, Syphilis, etc. ◦ SOPs for drawing, testing, processing, inventory control and lot traceability Dr. Arora - Good Manufacturing Practices

Bottom Line Document, Document!!! In FDA-Language: “If it is not documented. . . It did not happen!” or “It’s a rumor!” Dr. Arora - Good Manufacturing Practices

The Cardinal Rules of GMP l To err is human. ◦ To destroy the evidence is a felony l Maintain every record as if it were going to be reviewed by the FDA. l Your signature is precious ◦ Never sign, approve, or authorize anything you are not absolutely sure is correct. Dr. Arora - Good Manufacturing Practices