Good Manufacturing Practices and Instant GMP Software Orientation



























- Slides: 48

Good Manufacturing Practices and Instant. GMP™ Software Orientation Slides

Topics Overview • Purpose of GMPs • Quality Systems • GMP Process Flow Training Overview • Training Preparation • Scheduling Appointments Master Production Record Workflow • Materials • Products to be manufactured • Specifications • Master Production Records Batch Production Record Workflow • • • Purchasing Material Receipt Batch Production Inventory Summary

Instant. GMP Quick Facts • Cloud-based • Team of GMP manufacturing and quality experts used to guide software development • 21 CFR Part 11, GAMP 5 and FDA validation requirements met • Software has been in use since 2004

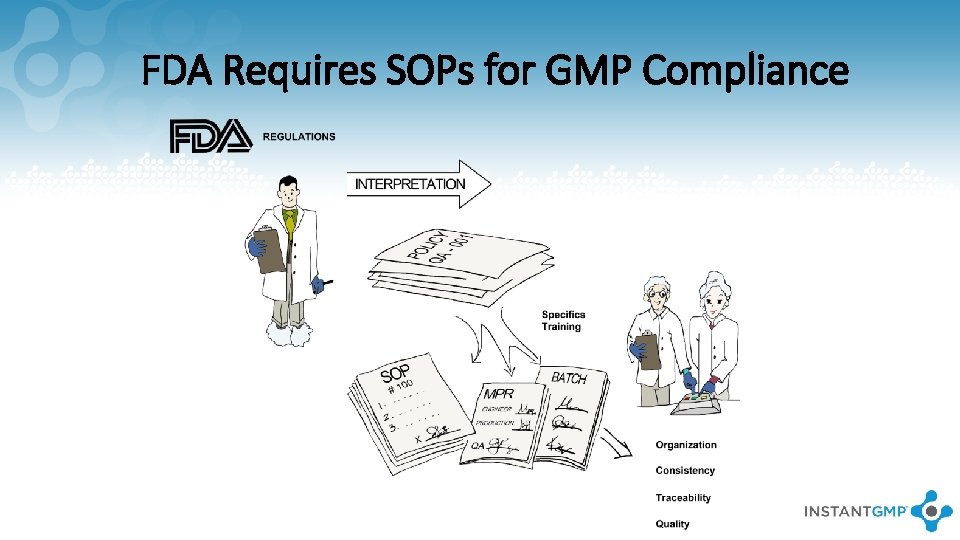
FDA Requires SOPs for GMP Compliance

Instant. GMP Consulting • Process Consulting • Help in making the transition from manual systems to electronic batch records • Create company specific Master Production Records (MPR) • Map company specific process flows • GMP Consulting • Assist in understanding GMP requirements • Help in developing a quality system for a manufacturing site • Advise on setting specifications and choosing tests and methods for raw materials and finished products • Teach GMP 101 course or annual GMP training

Who Must Follow c. GMPs?

Basic Tenants of GMPs FDA Mantra: “If it wasn’t documented, it wasn’t done!” • Instructions and procedures are clear and unambiguous • Manufacturing processes are clearly defined and controlled • Facilities are designed to minimize cross-contamination and mixups • Operators are trained • Records demonstrate that all required steps were taken • Distribution minimizes any risk • Manufacturing is governed by a Quality System consisting of Policies and SOPs 7

Purpose of Good Manufacturing Practices Following FDA requirements for Good Manufacturing Practices Gives you consistent, high quality products

Quality System • Quality = meeting specifications • Quality Control = testing to ensure specifications are met • Quality Assurance = review of testing results and promotion of practices intended to ensure quality • Quality System – policies, standard operating procedures and other documentation that define the practices needed to ensure quality • Good Manufacturing Practices lay the foundation for a quality system



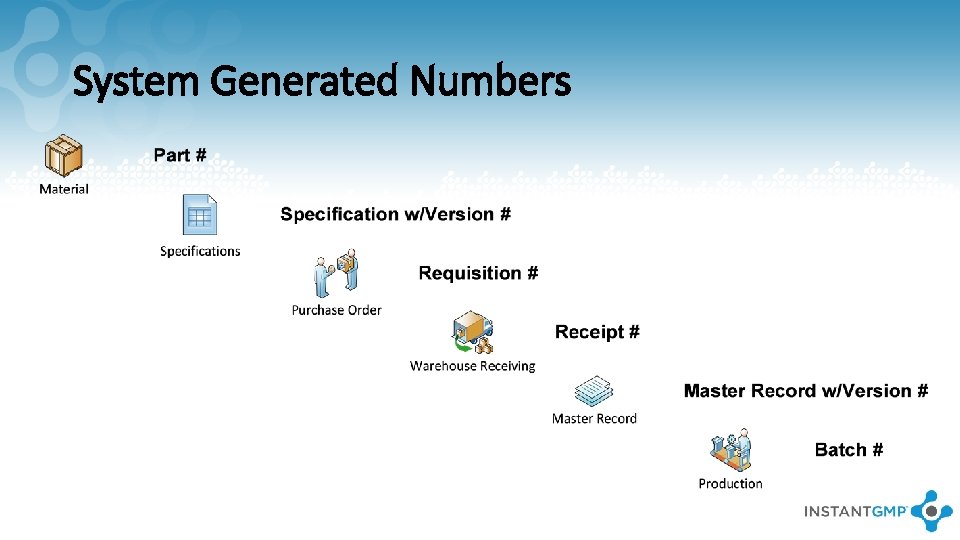
System Generated Numbers

Materials • Two classifications of materials: • Materials used in manufacturing • Raw Material • Incoming WIP • Other • Materials that are manufactured • Outgoing WIP • Finished Goods • Each Classification has a different input screen

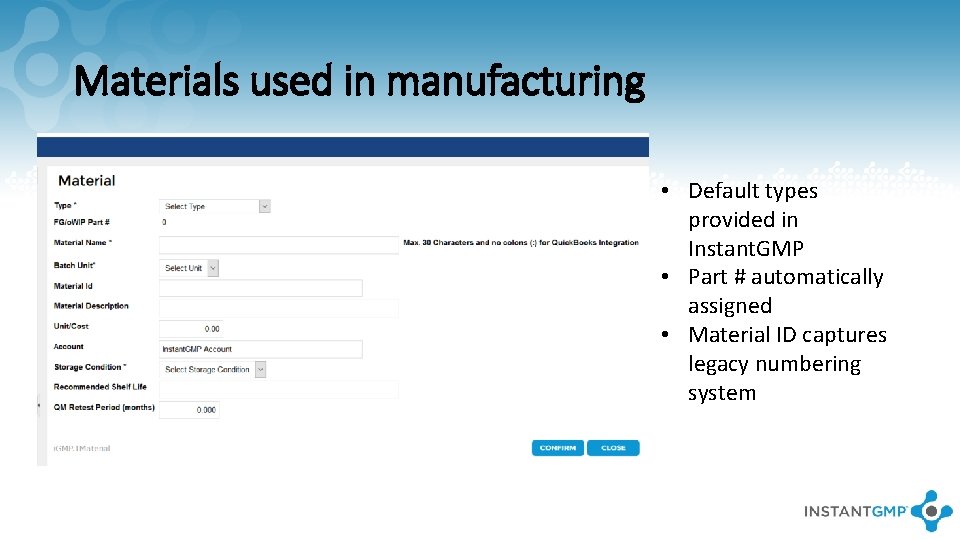
Materials used in manufacturing • Default types provided in Instant. GMP • Part # automatically assigned • Material ID captures legacy numbering system

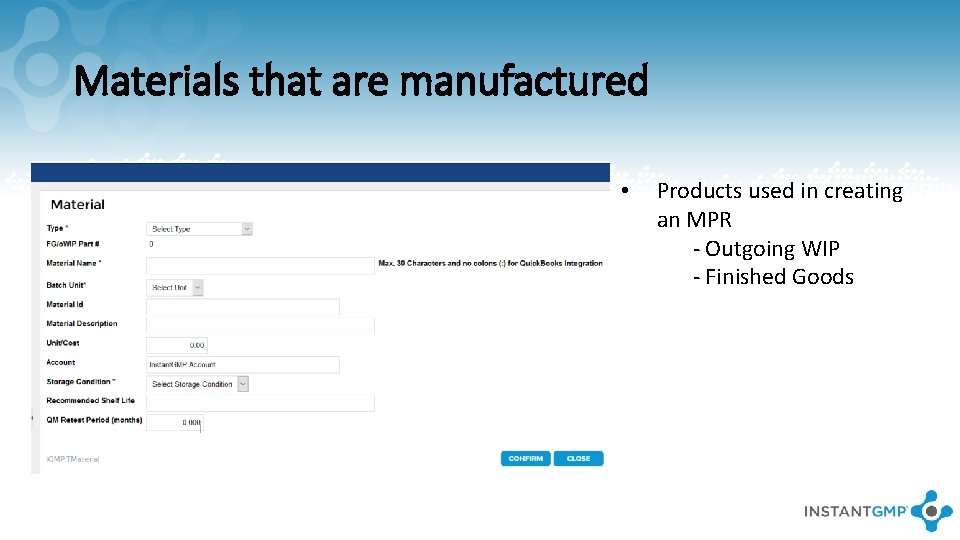
Materials that are manufactured • Products used in creating an MPR - Outgoing WIP - Finished Goods

Specifications • Make sure you are getting the right materials from your vendors • Allow you to verify identity, purity, strength and composition • Ensure you have the right ingredients in the product • Keep contaminants from adulterating a batch • Establish the basis of quality for your final product

Sp eci fic ati on s Ne ed ed For : • Components • In-process production • Labels and packaging • Finished batch of product • Packaging and labels • Received products

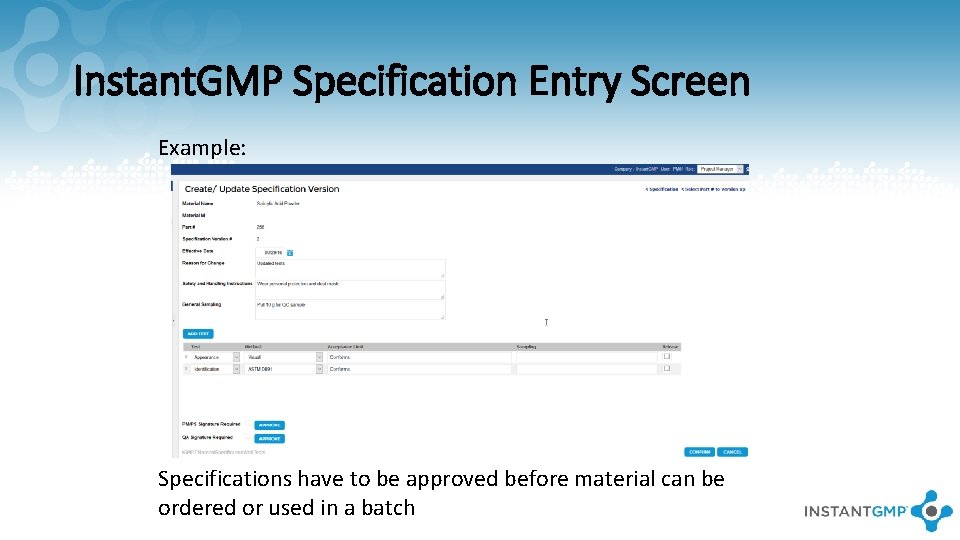
Instant. GMP Specification Entry Screen Example: Specifications have to be approved before material can be ordered or used in a batch

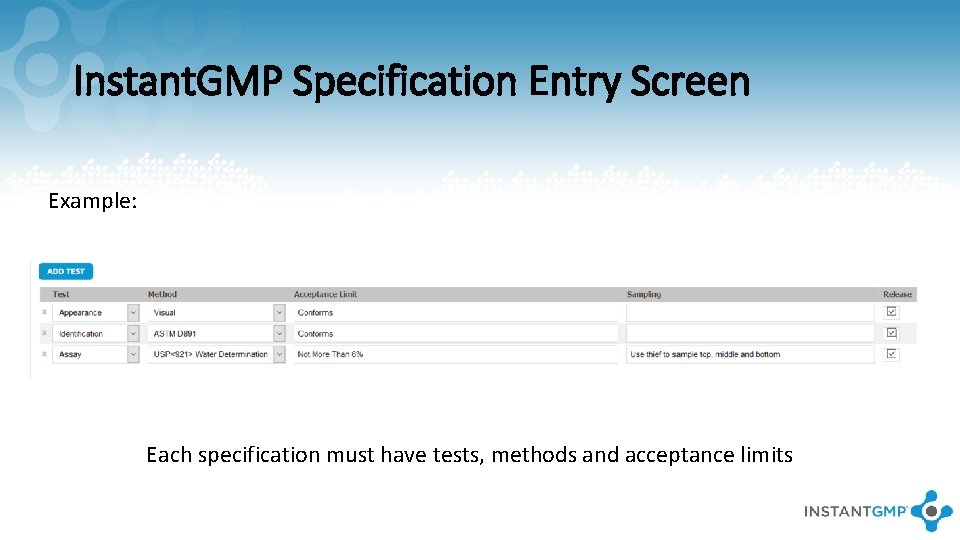
Instant. GMP Specification Entry Screen Example: Each specification must have tests, methods and acceptance limits

Projects

Add a WIP/FG to a Project 1. Click Add WIP/FG Part # 2. Select a Material Name Only FG and o. WIP (outgoing WIP) show in the list

Add a WIP/FG to a Project 3. Create a Product Name • Material Name + Strength + Container + Suffix must be a unique combination for each Product Name • Strength, Container and Suffix are optional

Add / Delete a WIP/FG in a Project • Multiple products can be added to any project • Product can be modified or deleted up until the product is used in an approved MPR

Creating a new MPR Definitions of “Theoretical Batch Yield” and “Batch Size” added • Project/Product/Client combination creates flexibility • Strength is inherited from Product Name • Batch Unit is inherited from Tmaterial • Unique for each MPR lineage of versions • • • Product Name/Strength Client Formulation ID Theoretical Batch Yield Batch Unit

MPR Versioning • Each new MPR version will be a copy that can be edited except for the following that will be kept constant: • • • Product Name/Strength Client Formulation ID Theoretical Batch Yield Batch Unit

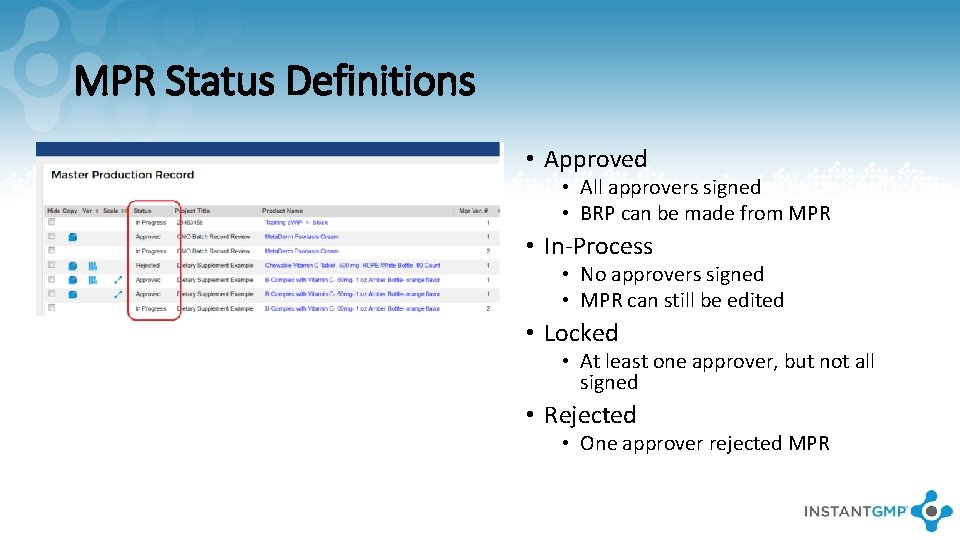
MPR Status Definitions • Approved • All approvers signed • BRP can be made from MPR • In-Process • No approvers signed • MPR can still be edited • Locked • At least one approver, but not all signed • Rejected • One approver rejected MPR

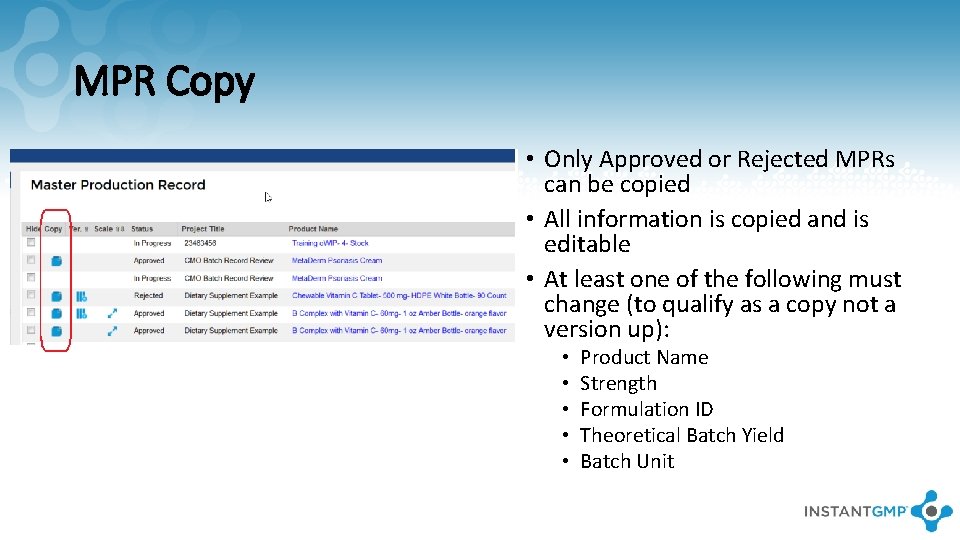
MPR Copy • Only Approved or Rejected MPRs can be copied • All information is copied and is editable • At least one of the following must change (to qualify as a copy not a version up): • • • Product Name Strength Formulation ID Theoretical Batch Yield Batch Unit

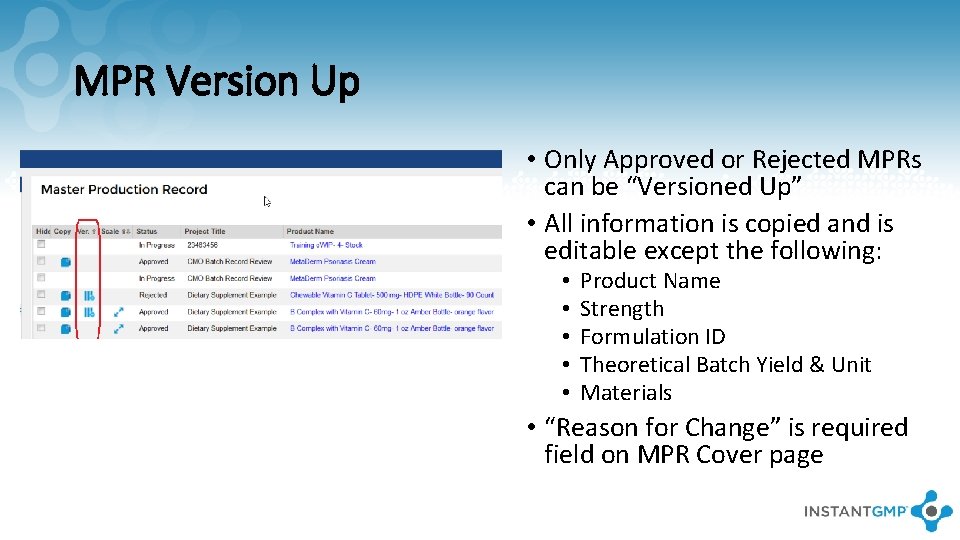
MPR Version Up • Only Approved or Rejected MPRs can be “Versioned Up” • All information is copied and is editable except the following: • • • Product Name Strength Formulation ID Theoretical Batch Yield & Unit Materials • “Reason for Change” is required field on MPR Cover page

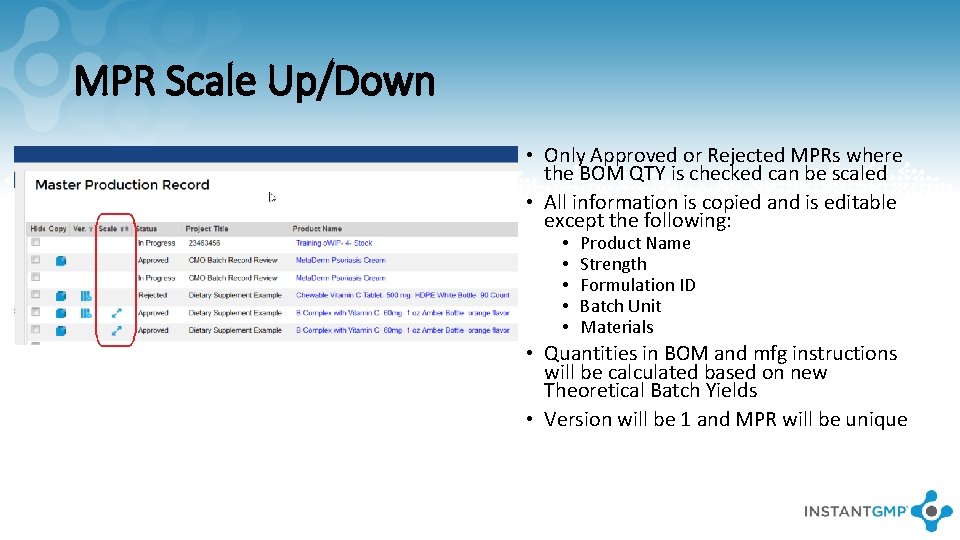
MPR Scale Up/Down • Only Approved or Rejected MPRs where the BOM QTY is checked can be scaled • All information is copied and is editable except the following: • • • Product Name Strength Formulation ID Batch Unit Materials • Quantities in BOM and mfg instructions will be calculated based on new Theoretical Batch Yields • Version will be 1 and MPR will be unique

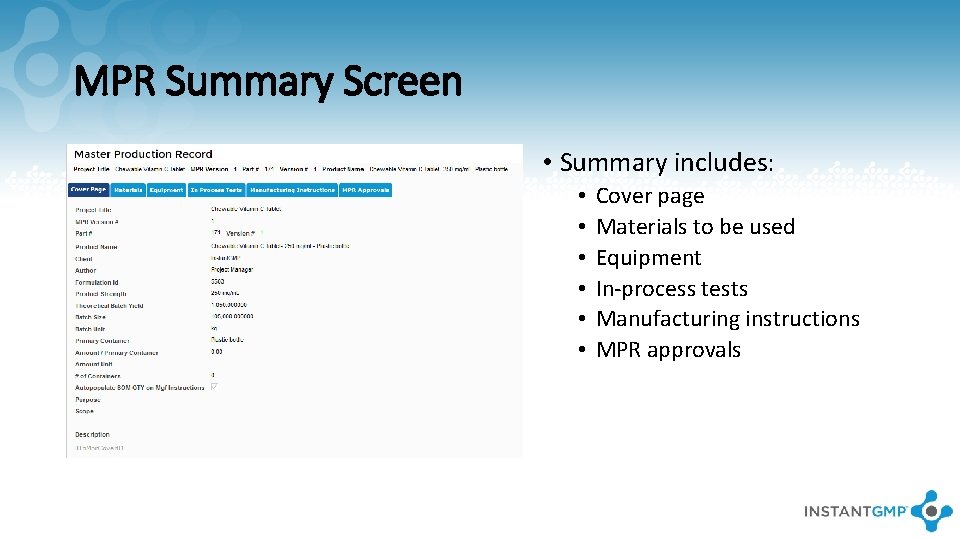
MPR Summary Screen • Summary includes: • • • Cover page Materials to be used Equipment In-process tests Manufacturing instructions MPR approvals


Good Manufacturing Practices and Instant. GMP™ Orientation Part Three: Batch Production Record

Good Manufacturing Practices and Instant. GMP™ Orientation Part Three: Batch Production Record

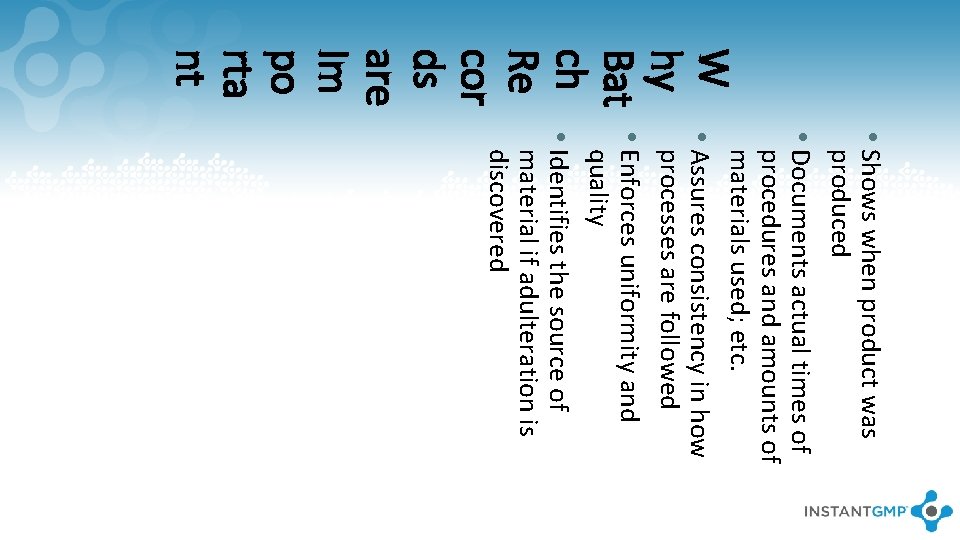
W hy Bat ch Re cor ds are Im po rta nt • Shows when product was produced • Documents actual times of procedures and amounts of materials used; etc. • Assures consistency in how processes are followed • Enforces uniformity and quality • Identifies the source of material if adulteration is discovered

Requisitions • Materials and components must come from qualified vendors • Materials and components must have specifications approved by the Quality Unit • All chemicals need a Material Safety Data Sheets (MSDS) on file • Certificate of Analysis (COA) is needed for each material to be used in manufacturing a product • Incoming materials must be same material and same grade as specified on purchase order

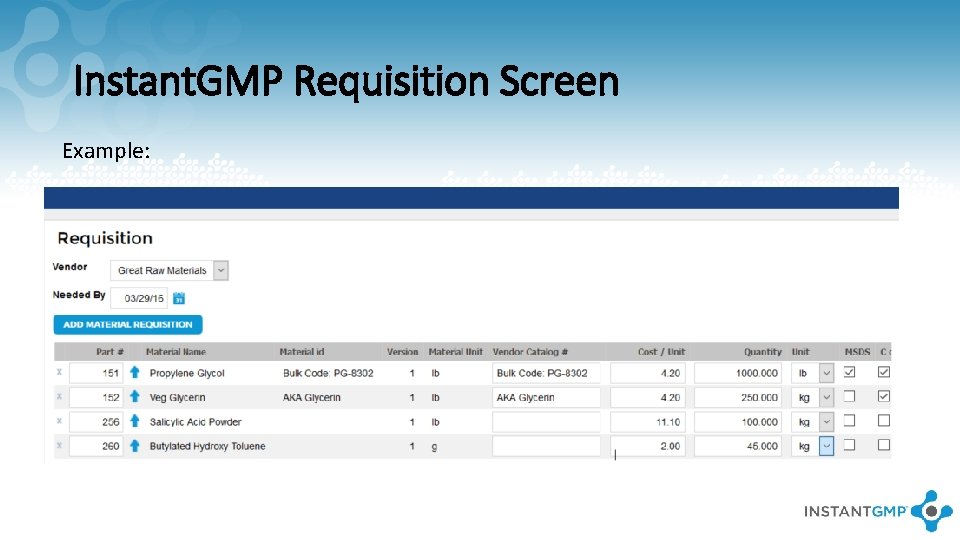
Instant. GMP Requisition Screen Example:

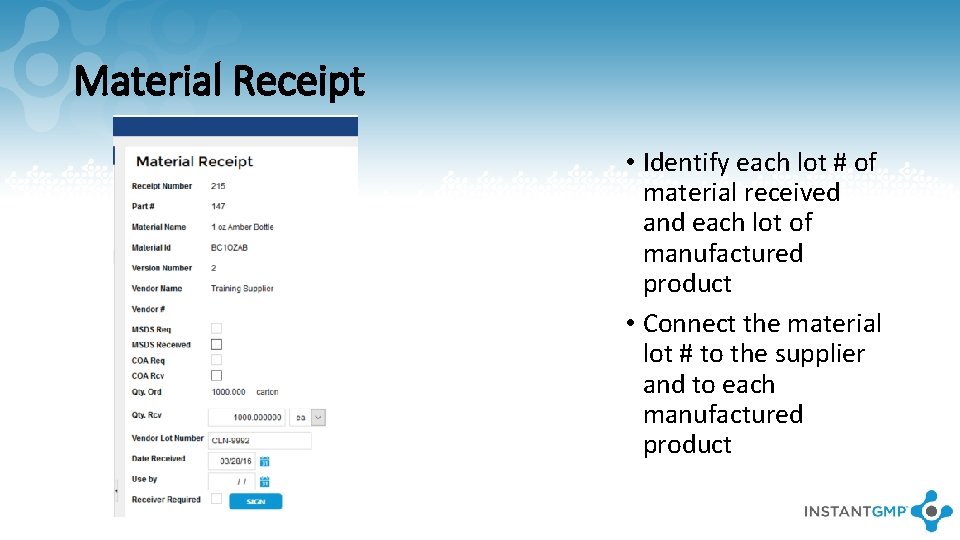
Material Receipt • Identify each lot # of material received and each lot of manufactured product • Connect the material lot # to the supplier and to each manufactured product

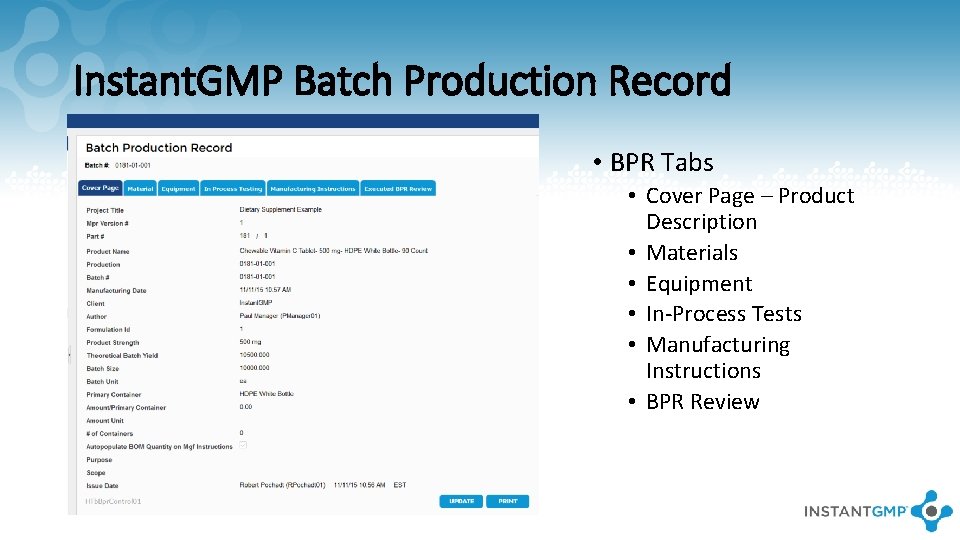
Instant. GMP Batch Production Record • BPR Tabs • Cover Page – Product Description • Materials • Equipment • In-Process Tests • Manufacturing Instructions • BPR Review

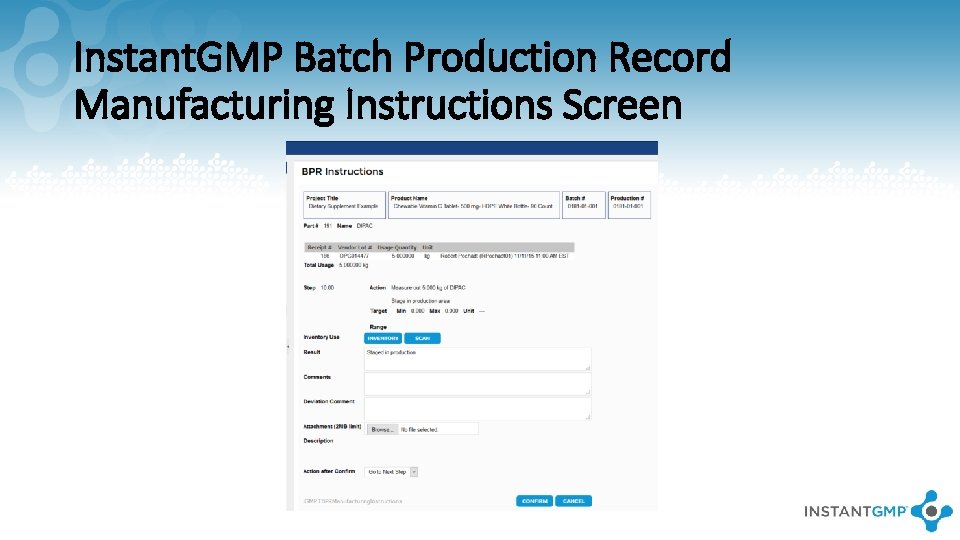
Instant. GMP Batch Production Record Manufacturing Instructions Screen

Distribution Procedures • Products should only be shipped out for distribution after they have been released by the quality unit • Products should be transported in a way that maintains their quality • Special transport or storage conditions should be stated on the label • A system should readily permit the recall of a product if needed

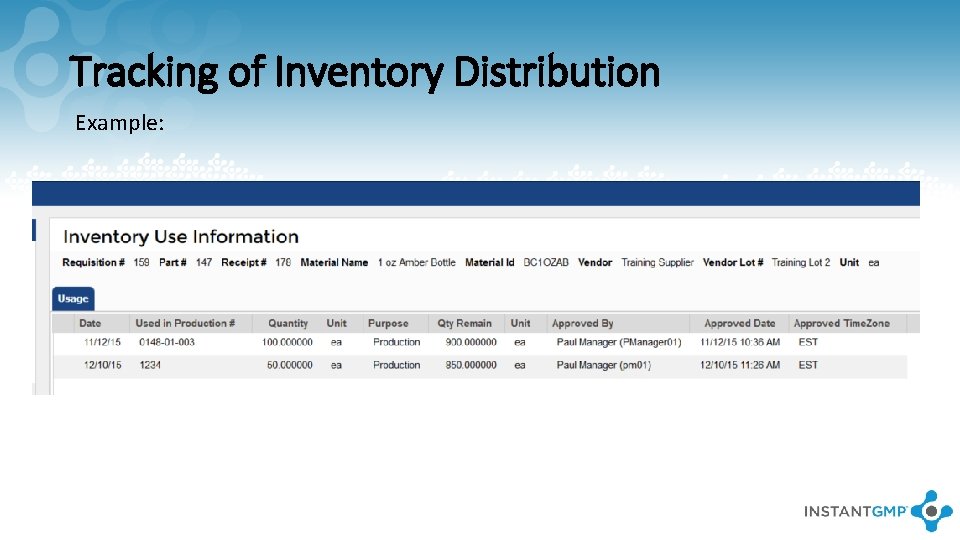
Tracking of Inventory Distribution Example:



Summary • • Good Manufacturing Practices are complex and thorough Wide array of regulatory requirements need to be followed Following GMPs gives you high quality products Instant. GMP guides you through the GMP manufacturing process Remember the FDA mantra: “If it wasn’t documented, it wasn’t done!”

Next – Software Training • 4 -8 hour interactive introduction to the Instant. GMP software • Familiarizes the Project Manager (PM) and Quality Manager (QM) with the workflows • Designate one Project Manager and one Quality Manger who can commit to the full training program • Additional paid training is available

Getting Ready for Software Training • Download the Join. me desktop App from http: //www. Instant. GMP. com/joinme • Note: Make sure to use the computer that will be used during software training. • To make your Software Training appointment, go to: http: //www. instantgmp. com/support/appointment-request

Find articles and videos on c. GMP compliance and quality in the Resource Center at Instant. GMP. com
