Good Hygiene Practices along the coffee chain Module









- Slides: 12

Good Hygiene Practices along the coffee chain Module 4. 10 Establish Corrective Actions (Task 10 / Principle 5)

Objectives and contents § Objectives § To enable trainees to establish effective procedures for corrective actions when there are deviations from critical limits § Contents § What is ‘process deviation’ and ‘loss of control’? § Scope of deviation procedures / corrective action § Deviation and corrective action records 2 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

Corrective action. . . any action to be taken when the results of monitoring at the CCP indicate a loss of control Where loss of control is § A deviation from a critical limit 3 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

Proactive nature of HACCP § In HACCP plans deviation procedures are pre-determined and documented actions to be implemented in case of a deviation § Control of non-compliant product § Addressing source of problem within the process § Trend of deviation from operating limits adjustments to prevent loss of control 4 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

Corrective actions § Depending on the process, several deviations may be possible at a CCP § More than one corrective action may be required for each CCP § Individuals responsible for monitoring must be trained to adequately perform corrective actions when necessary 5 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

Product deviation – controlling non-compliant product § Process deviation is ‘failure to meet critical limit’ § A deviation implies that a safety breach has occurred Immediate action § Activates a system that identifies, marks and isolates all product affected over the deviation period § Producer must maintain control of suspect batch pending evaluation Deferred action § The evaluation establishes the status of the batch regarding safety - to release, reprocess, or destroy 6 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

Deviation procedures – controlling cause of deviation § Investigation to determine the cause of deviation § Determine if measures can be taken to prevent recurrence of the deviation § Possible re-evaluation of aspects of the HACCP plan or related hazard analysis § Verify that the corrective action was effective 7 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

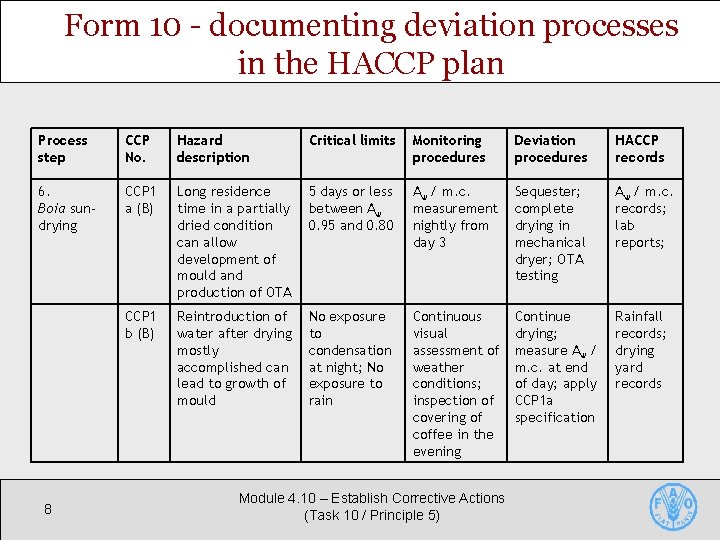
Form 10 - documenting deviation processes in the HACCP plan Process step CCP No. Hazard description Critical limits Monitoring procedures Deviation procedures HACCP records 6. Boia sundrying CCP 1 a (B) Long residence time in a partially dried condition can allow development of mould and production of OTA 5 days or less between Aw 0. 95 and 0. 80 Aw / m. c. measurement nightly from day 3 Sequester; complete drying in mechanical dryer; OTA testing Aw / m. c. records; lab reports; CCP 1 b (B) Reintroduction of water after drying mostly accomplished can lead to growth of mould No exposure to condensation at night; No exposure to rain Continuous visual assessment of weather conditions; inspection of covering of coffee in the evening Continue drying; measure Aw / m. c. at end of day; apply CCP 1 a specification Rainfall records; drying yard records 8 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

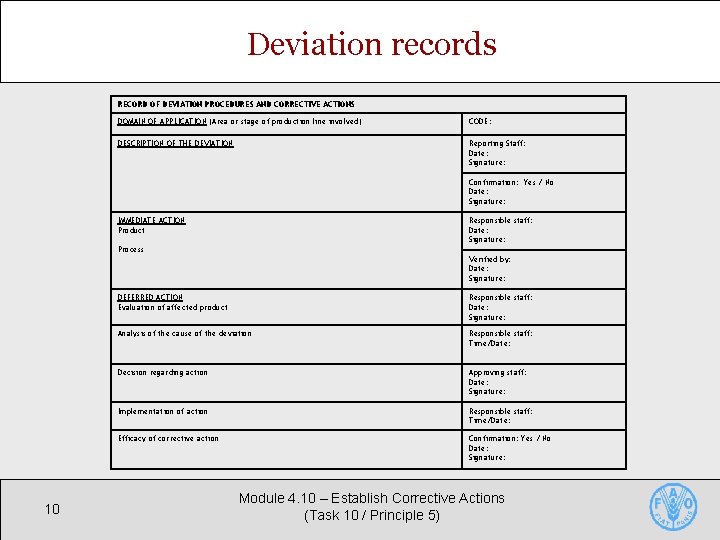
Deviation records § Accurate records of deviations are essential § Demonstrate effective control of affected products § Facilitate system improvements § Deviation records include § Product affected • Product / code; date produced / held / released; reason for holding; amount of product affected; details of evaluation; signature of staff taking or approving action § Corrective action • Cause of deviation; action taken; follow-up 9 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

Deviation records RECORD OF DEVIATION PROCEDURES AND CORRECTIVE ACTIONS DOMAIN OF APPLICATION (Area or stage of production line involved) CODE: DESCRIPTION OF THE DEVIATION Reporting Staff: Date: Signature: Confirmation: Yes / No Date: Signature: IMMEDIATE ACTION Product Responsible staff: Date: Signature: Process Verified by: Date: Signature: 10 DEFERRED ACTION Evaluation of affected product Responsible staff: Date: Signature: Analysis of the cause of the deviation Responsible staff: Time/Date: Decision regarding action Approving staff: Date: Signature: Implementation of action Responsible staff: Time/Date: Efficacy of corrective action Confirmation: Yes / No Date: Signature: Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

Dealing with process deviations § Deviation procedures cannot be generalized they depend on products and production methods § However, identification and isolation of affected product for evaluation is the first step § Deviations may arise from mechanical faults, procedural oversights or, in some cases, external specification violation § Follow-up might include plant renewal, staff retraining, education of suppliers 11 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)

Summary § Deviation and corrective action procedures § Documenting corrective actions in a HACCP plan § Deviation records Next module: Establish verification procedures 12 Module 4. 10 – Establish Corrective Actions (Task 10 / Principle 5)