Good Governance for Medicines Programme Dr Ccile Mac










- Slides: 13

Good Governance for Medicines Programme Dr Cécile Macé EMP/MPC 1| WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

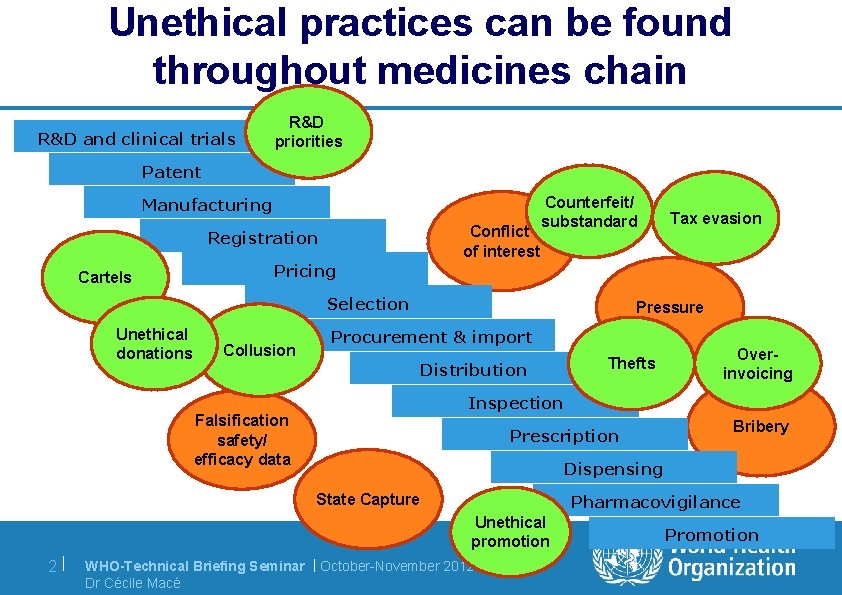
Unethical practices can be found throughout medicines chain R & D a n d c l i n i c a l t ri a l s R&D priorities P a t en t M a n u f a c t u ri n g Conflict of interest R eg i s t ra t i o n Cartels Counterfeit/ substandard P ri c i n g S el ec t i o n Unethical donations Collusion Pressure P ro c u rem en t & i m p o rt Di s t ri b u t i o n Thefts Overinvoicing I n s p ec t i o n Falsification safety/ efficacy data P res c ri p t i o n Bribery Di s p en s i n g State Capture P h a rm a c o v i g i l a n c e Unethical promotion 2| Tax evasion WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé P ro m o t i o n

Why a Good Governance for Medicines Programme? l To improve transparency, accountability, effective, efficient and ethical management of pharmaceutical systems l To improve the health service delivery to the population in countries through improved access to quality-assured medicines and rational use l To avoid wastage or misuse of public or donor funding in the pharmaceutical sector l To improve public trust and confidence on health system l To support countries to identify, prioritize and mitigate risks l To develop guidance to support countries in improving good governance in the pharmaceutical system 3| WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

WHO Good Governance for Medicines Programme: l Goal – To contribute to health systems strengthening and to prevent corruption by promoting good governance in the pharmaceutical sector l Specific objectives – To raise awareness on the impact of corruption in the pharmaceutical sector and bring this to the national health policy agenda – To increase transparency and accountability in medicine regulatory and supply management systems – To promote individual and institutional integrity in the pharmaceutical sector – To institutionalize good governance in pharmaceutical systems by building national capacity and leadership 4| WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

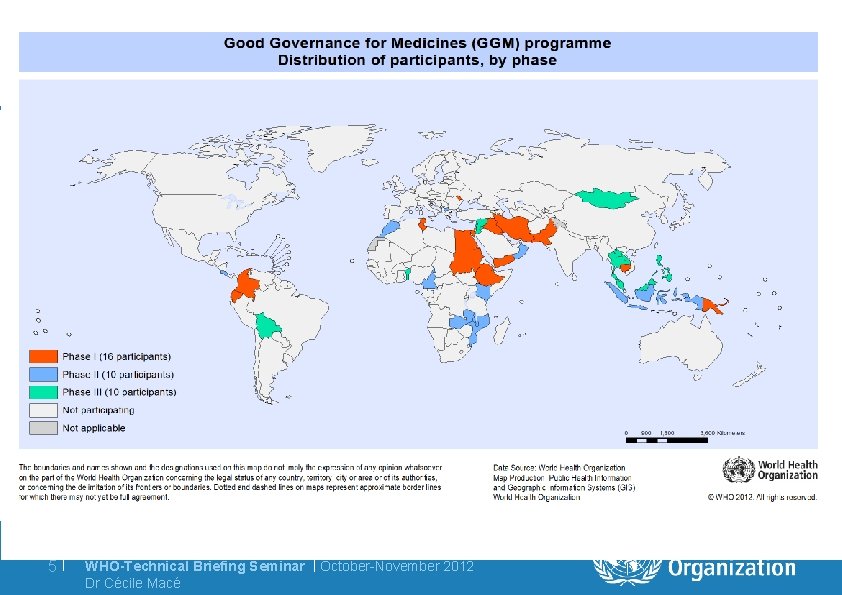
5| WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

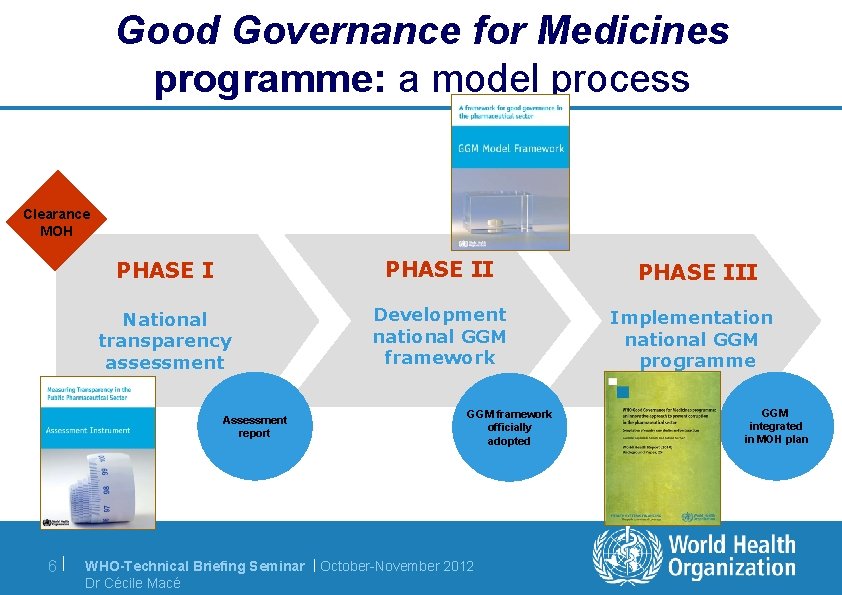
Good Governance for Medicines programme: a model process Clearance MOH PHASE II National transparency assessment Development national GGM framework Assessment report 6| GGM framework officially adopted WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé PHASE III Implementation national GGM programme GGM integrated in MOH plan

Achievements in countries Increased awareness of impact of corruption in the Pharmaceutical Sector and Importance of having Good Governance and Transparency 1. National Assessments done and published 2. GGM incorporated in National Health Agenda by nominating a task force and steering committee to work on framework 3. Increase in political will to implement GGM 4. Collaboration between various stakeholders (Mo. H, other ministries, anti-corruption commission, NGOs, private sector…) 7| WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

Achievements in countries Increased Transparency and Accountability in Medicine Regulatory and Supply Systems 1. Various laws, regulations, SOPs created or reviewed/updated 2. Management of conflicts of interest put in place for various committees 3. Information publicly available to increase transparency 4. Whistle-blower protection bill passed, increase in number of corruption cases investigated 5. Increased accessibility of medicines at lower costs 6. Appeal mechanism put in place 8| WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

Achievements in countries Increased promotion of individual and institutional integrity in the pharmaceutical sector 1. National GGM Framework developed, adopted and published 2. Creation of Code of conduct for people working in the public pharmaceutical sector 3. Continuous training workshops on ethical leadership and GGM at national and regional level 4. Continuous collaboration with other stakeholders 5. GGM included in the curricula of phamacy students 9| WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

Achievements in countries Institutionalization of GGM 1. In Mongolia, the Mo. H has designated by law in 2010 the Drug Regulatory Agency to implement GGM 2. In Philippines, the GGM team in the Mo. H is having a specific budget to conduct activities 3. Still in progress in other countries… 10 | WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

Common challenges faced in implementation l Cultural and behavioural: resistance to change, passive attitude or tolerance l Political: instability, change in government l Managerial: lack staff, rotation, lack of financial resources l Structural: more difficult if basic systems not in place l Technical: integration in day to day affairs, new subject, access to legislation documents l Time: workload, other priorities; GGM not a priority 11 | WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

Key observations and lessons learnt 1. Great interest in subject area 2. A dedicated and motivated national team to tackle the issue 3. Involvement of high-level and technical officials essential 4. Collaboration with key stakeholders 5. Promotion of integrity together with legislative reforms 6. Timeframe different between countries 7. Institutionalization needed for sustainability 12 | WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé

Thank you! macec@who. int http: //www. who. int/medicines/ggm/en/index. html 13 | WHO-Technical Briefing Seminar | October-November 2012 Dr Cécile Macé