Good Distribution Practices Securing The Supply Chain Michael
Good Distribution Practices Securing The Supply Chain Michael H. Anisfeld Globepharm Consulting Sindusfarma, Brasilia, August 2014

QUALITY: FROM START TO FINISH GMP GLP GCP GDP G 2

GDPs are Concerned With Product Integrity Counterfeiting Temperature Degradation Theft / Tampering Know Your Supplier Stock Rotation Sanitation Record Keeping Supplier manufactures your product from 09: 00 – 17: 00 Supplier continues the manufacturing line for his benefit from 17: 00 – 23: 00 Supplier establishes off-site production using your raw materials
Supply Chain From Field To You
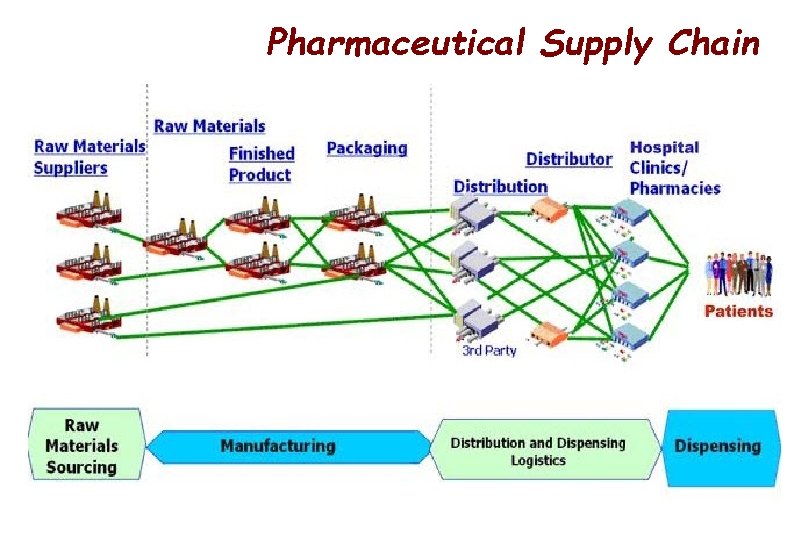
Pharmaceutical Supply Chain
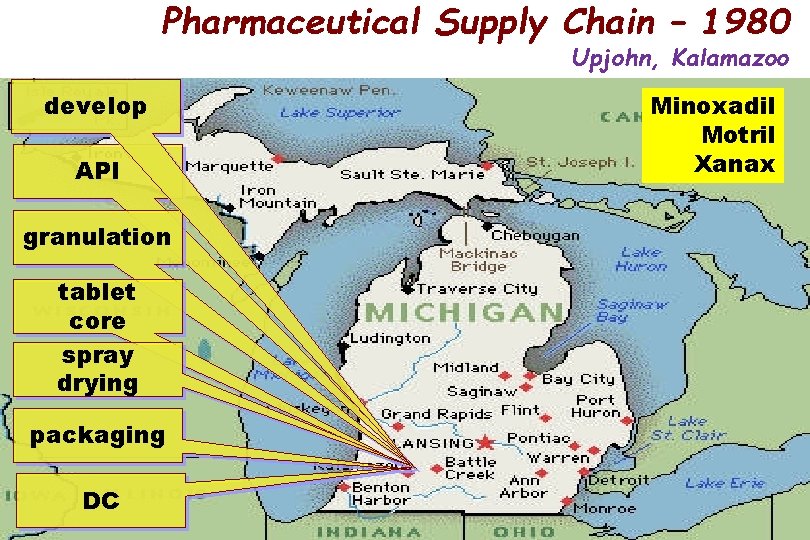
Pharmaceutical Supply Chain – 1980 Upjohn, Kalamazoo deve. Iop API granulation tablet core spray drying packaging DC Minoxadil Motril Xanax
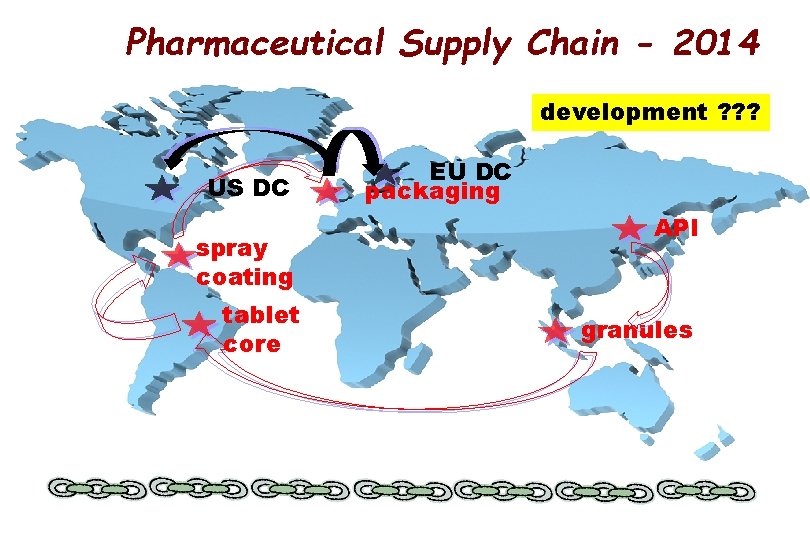
Pharmaceutical Supply Chain - 2014 development ? ? ? US DC spray coating tablet core EU DC packaging API granules
Good Distribution Practices

GDP + GSP GDPs exist in over 20 countries, but almost all are local language versions of WHO GDPs Good Storage Practices (GSP) In the 1990 s several countries developed “Good Storage Practices” (GSP), but these have mainly been merged with Good Distribution Practices into a single guideline
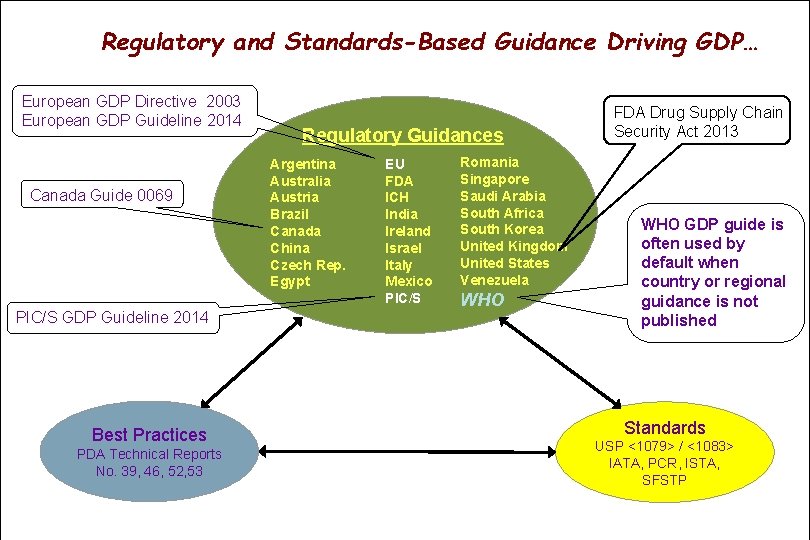
Regulatory and Standards-Based Guidance Driving GDP… European GDP Directive 2003 European GDP Guideline 2014 Canada Guide 0069 PIC/S GDP Guideline 2014 Best Practices PDA Technical Reports No. 39, 46, 52, 53 Regulatory Guidances Argentina Australia Austria Brazil Canada China Czech Rep. Egypt EU FDA ICH India Ireland Israel Italy Mexico PIC/S Romania Singapore Saudi Arabia South Africa South Korea United Kingdom United States Venezuela WHO FDA Drug Supply Chain Security Act 2013 WHO GDP guide is often used by default when country or regional guidance is not published Standards USP <1079> / <1083> IATA, PCR, ISTA, SFSTP

H : W e l g Goo O“ d Goo Dis t tio ribu n. P ice t c ra s”

Good Distribution Practices (GDP) Definition: That part of Quality Assurance that ensures that the quality of a pharmaceutical product is maintained by means of adequate control of the numerous activities which occur during the distribution process as well as providing a tool to secure the distribution system from unapproved, illegally imported, stolen, counterfeit, substandard, adulterated, and/or misbranded pharmaceutical products.

World Health Organization GDP Key Concerns: Integrity of the Supply Chain – Counterfeits Maintaining the Cold Chain Sanitation Recordkeeping

EU (GDP Guide) Country Regulations and Guidance: Continued Development Ensure storage conditions at all times controlled temperature products (15 -25 o. C) should be transported appropriately Brazil / Saudi Arabia (very similar) Electronic data logger in every shipment For import release, all incoming biological products must have temperature monitoring records showing proper storage temperatures India Constant temperature monitoring Refrigerated vans should be qualified. Qualification should be done by keeping sufficient temperature monitors to cover all parts of the van Product Serialization (at various states of progress) United States, Europe, Turkey, Brazil and India

Quality Agreements tool for communication and compliance Incorporate applicable regulation, standard, and best practice guidances Integrate competing perspectives: Product Requirements vs. Service Levels Quality Agreement specific to transport
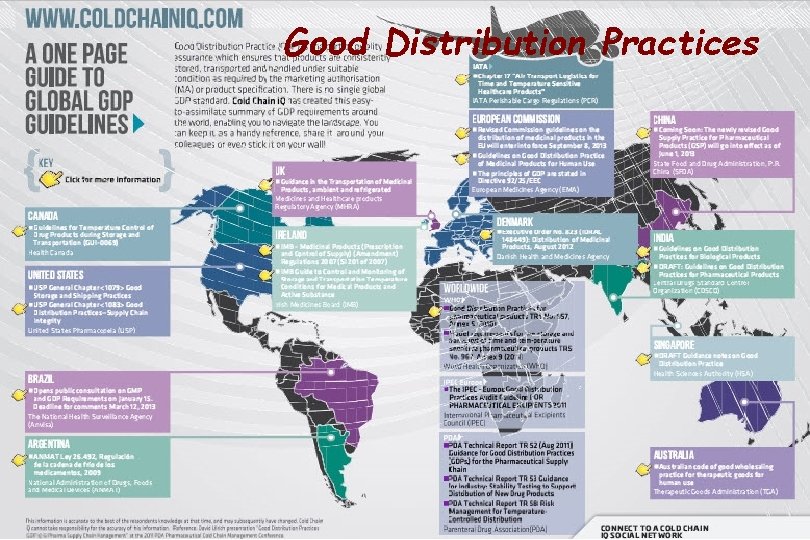
Good Distribution Practices

Product Integrity Counterfeits

What Are We Talking About Piracy Counterfeit Software CDs DVDs T-Shirts Sunglasses Watches Money Sports Shoes Medicinal Products USA: illicit DVD downloading FDA Commissioner Dr. Margaret Hamburg st time: penalties 1 up to five for years' jail + “Current peddling (in 2012 Congressional testimony) fine up to $ 250, 000 fake medicines were the same as those for $ 500, 000 punishments for illicit drug sellers. nd time: fake 2 selling up tohandbags, ten years’and jail weaker + fine up to than

19


Counterfeit Medicine A counterfeit medicine is one which is deliberately and fraudulently mislabelled with respect to identity and/or source. Counterfeiting can apply both to branded or generic products with the correct ingredients or with the wrong ingredients, without active ingredients, with insufficient active ingredients or false packaging.

Counterfeit Medicine The definition of ‘counterfeit medicinal products’ should be more clearly stated. . . Proposal: A ‘counterfeit medicinal product’ is a product that is not the medicinal product which it pretends to be. This means that every medicinal product in the market that does not comply with its Marketing Authorization is a counterfeit medicine – regardless if the non-compliance was caused by fraud, (gross) negligence or accident
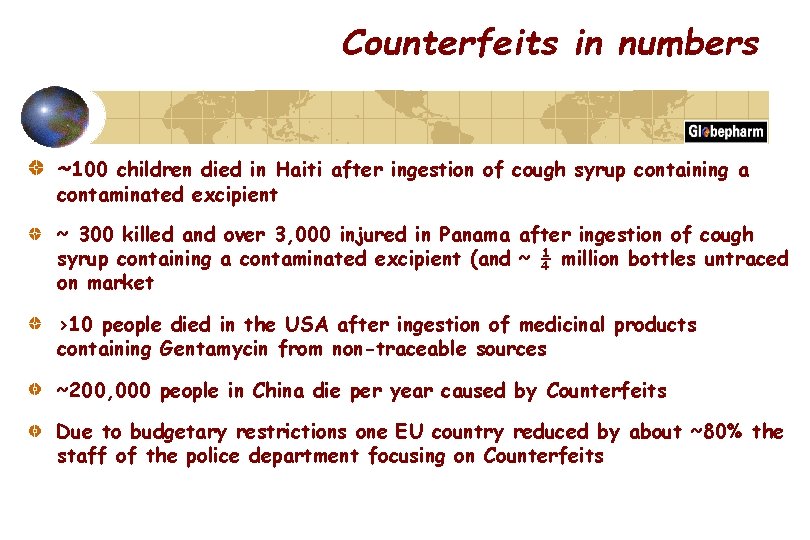
Counterfeits in numbers ~100 children died in Haiti after ingestion of cough syrup containing a contaminated excipient ~ 300 killed and over 3, 000 injured in Panama after ingestion of cough syrup containing a contaminated excipient (and ~ ¼ million bottles untraced on market >10 people died in the USA after ingestion of medicinal products containing Gentamycin from non-traceable sources ~200, 000 people in China die per year caused by Counterfeits Due to budgetary restrictions one EU country reduced by about ~80% the staff of the police department focusing on Counterfeits

Pharmaceuticals World Market IMS - 2012 24

Counterfeiting is big business: 8 -15% of all pharmaceutical sales worldwide no country is immune 25
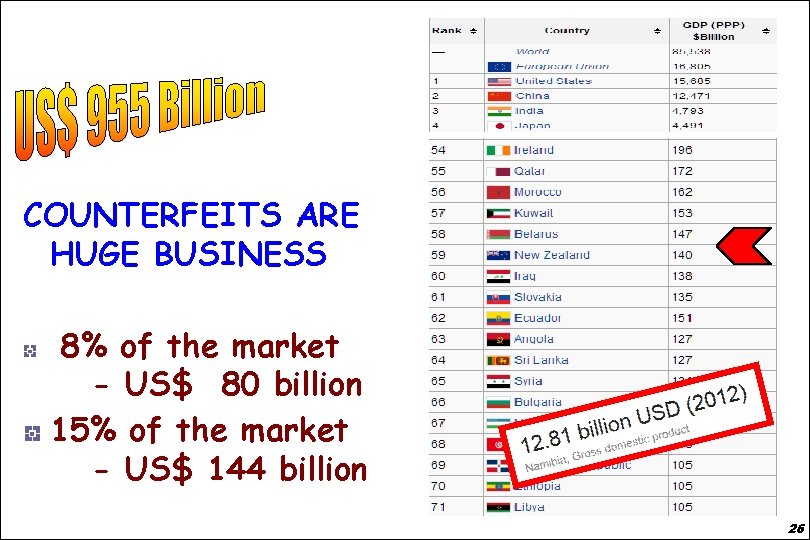
Counterfeit Drugs COUNTERFEITS ARE HUGE BUSINESS 8% of the market - US$ 80 billion 15% of the market - US$ 144 billion 26
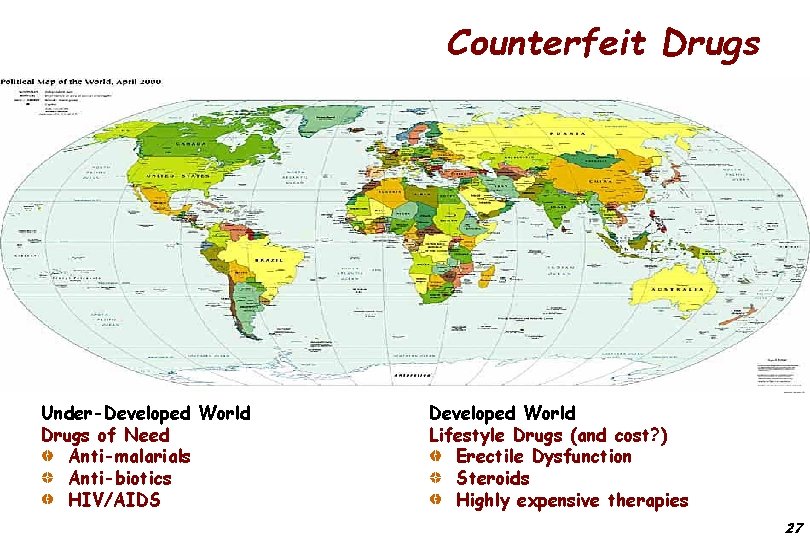
Counterfeit Drugs Under-Developed World Drugs of Need Anti-malarials Anti-biotics HIV/AIDS Developed World Lifestyle Drugs (and cost? ) Erectile Dysfunction Steroids Highly expensive therapies 27
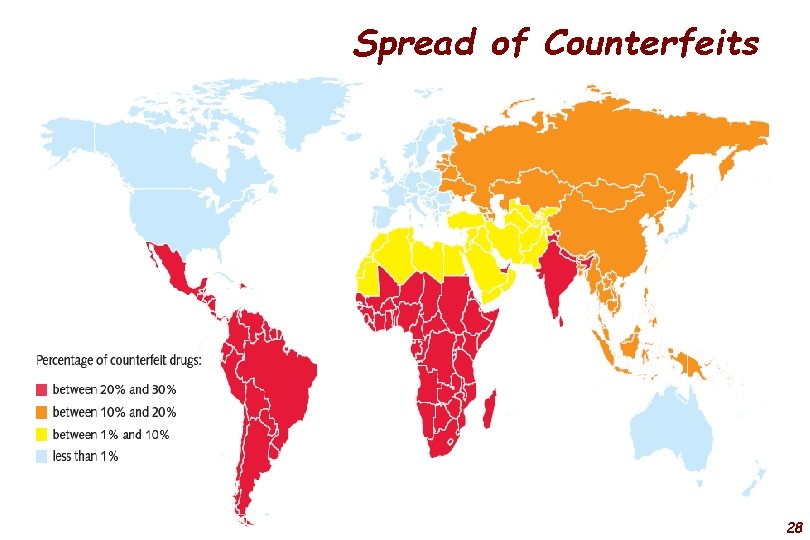
Spread of Counterfeits 28
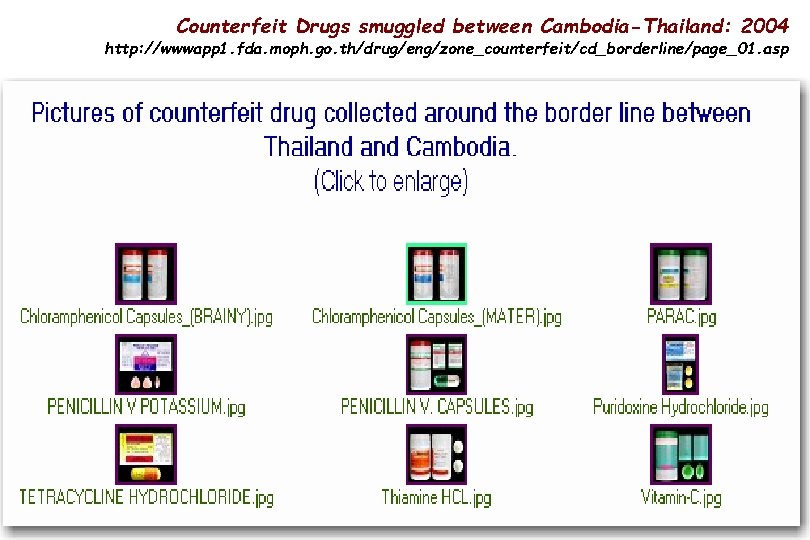
Counterfeit Drugs smuggled between Cambodia-Thailand: 2004 http: //wwwapp 1. fda. moph. go. th/drug/eng/zone_counterfeit/cd_borderline/page_01. asp 29

Vietnam, 2012 Real (left) vs. Fake Medication

North American Pharmaceutical Market Combivir (lamivudine) Gamimune (immune globulin) Neupogen (filgrastim) Nutropin (somatropin) Oxycontin (oxycodone) Procrit (epoetin) Serostim (somatropin) Viagra (sildenafil) Zyprexa (olanzipine) 31
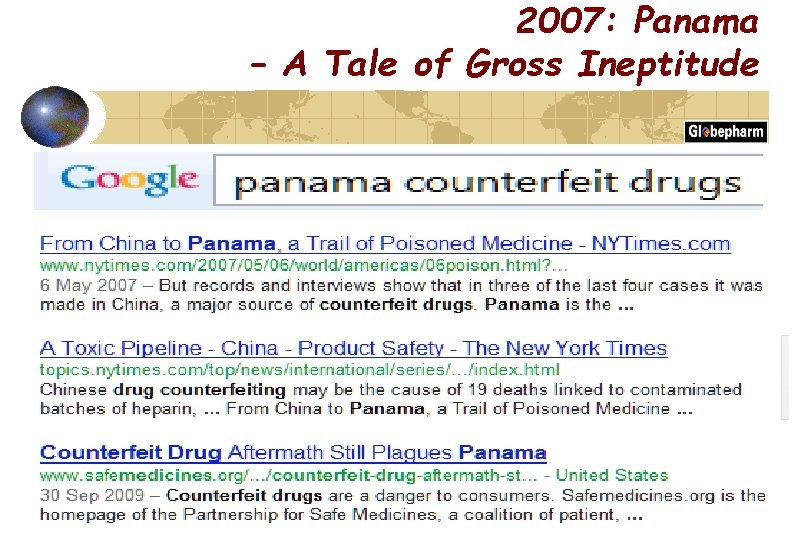
2007: Panama – A Tale of Gross Ineptitude
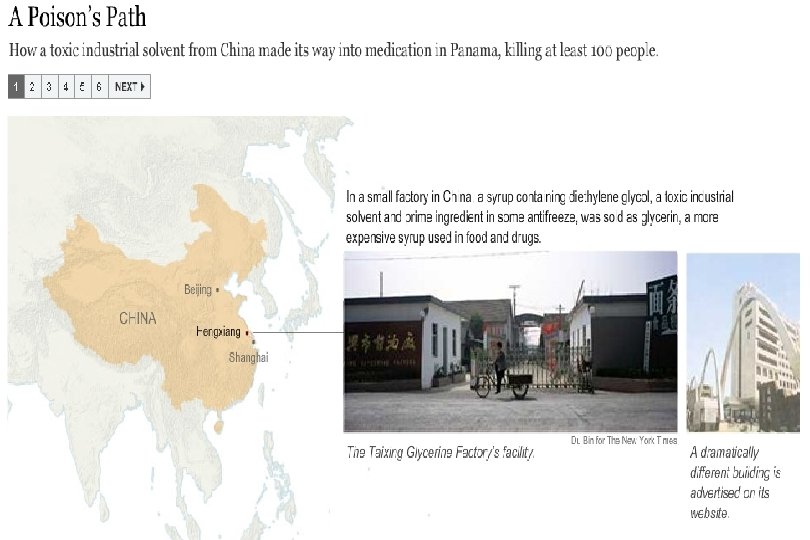
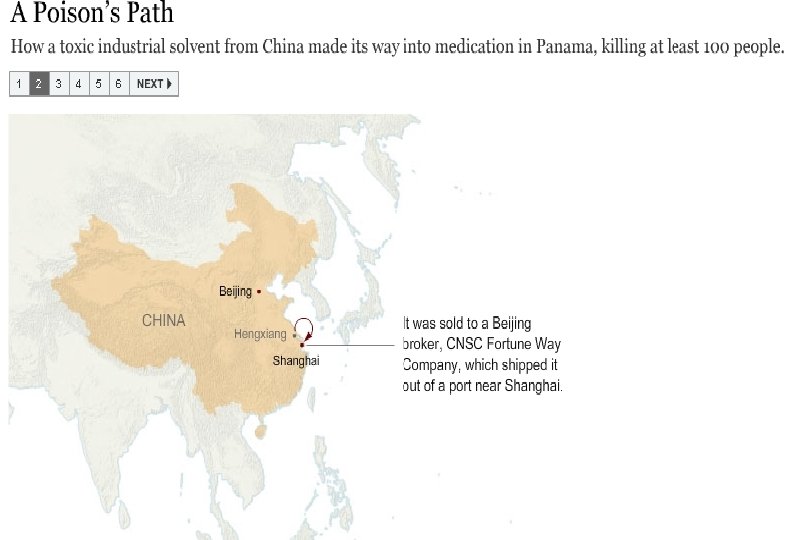
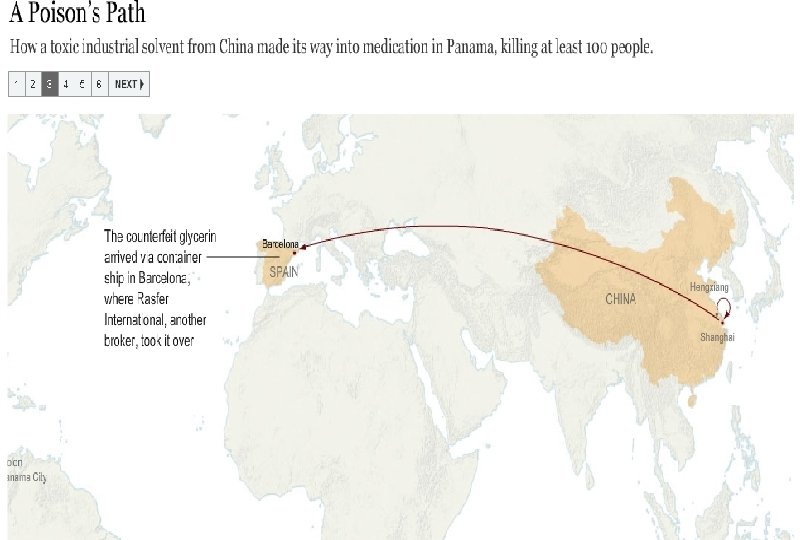
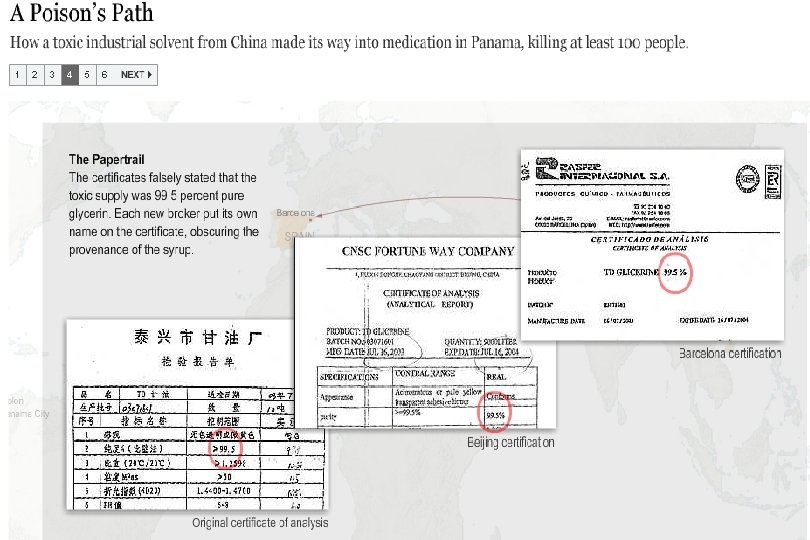
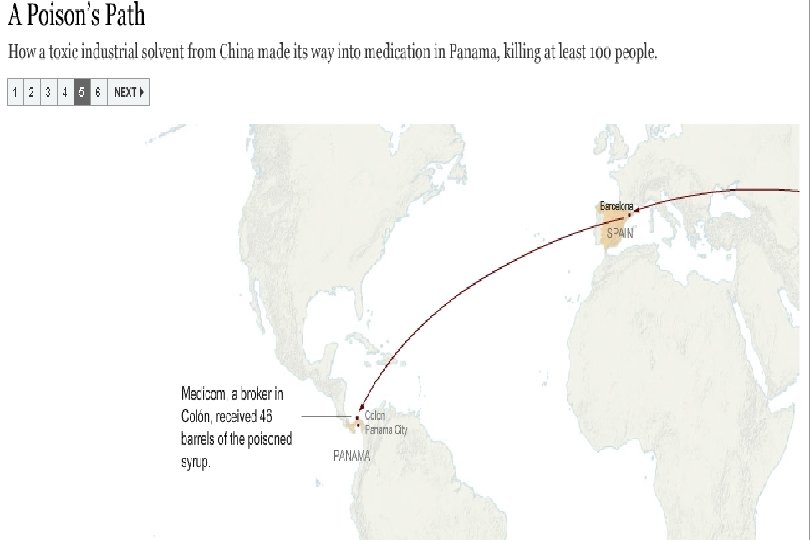
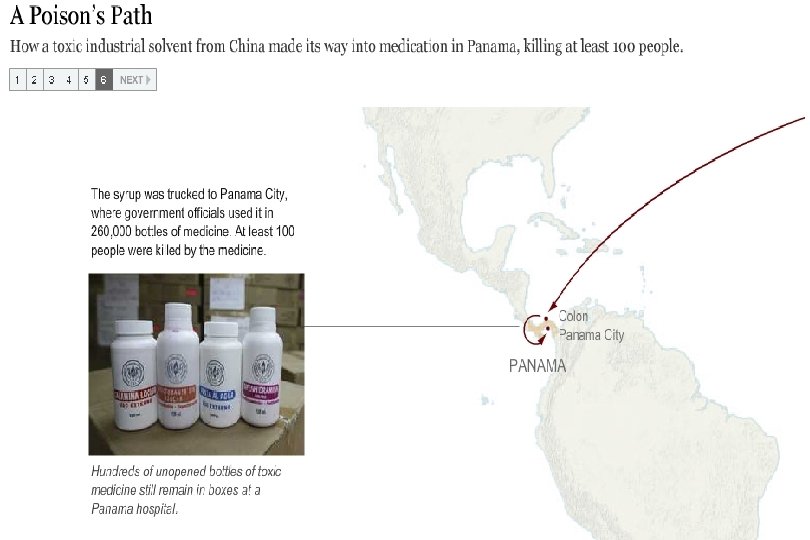

Preventable Deaths About 300 people have died in Panama, and over 3, 000 injured due to contaminated cough syrup. Deaths and injuries that could have easily been prevented by following GMPs: Purchase from audited raw material sources Purchase directly from the supplier, not through brokers Performing raw material identity and monograph testing Having effective recall traceability

The Problem Incompetent Manufacturing Lack of Governmental Controls Where Controls Exist - Lack of Effective Enforcement 40

We Cannot Keep Up! www. fip. org 41

Types of Counterfeiting a) A perfect copy of a drug with the same active ingredient and identical packaging. b) Counterfeits in packaging identical to that of a branded product. The drug usually includes the indicated active ingredient, but in insufficient quality or quantity. c) A product looks like the originator drug but contains no active ingredient. d) The counterfeit drug contains substances that are unhealthily or poisonous and leads to physical harm or death 42
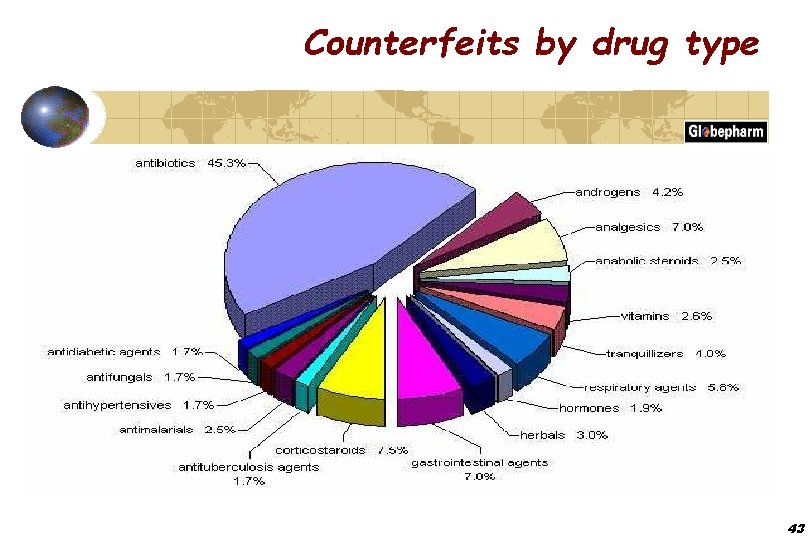
Counterfeits by drug type 43
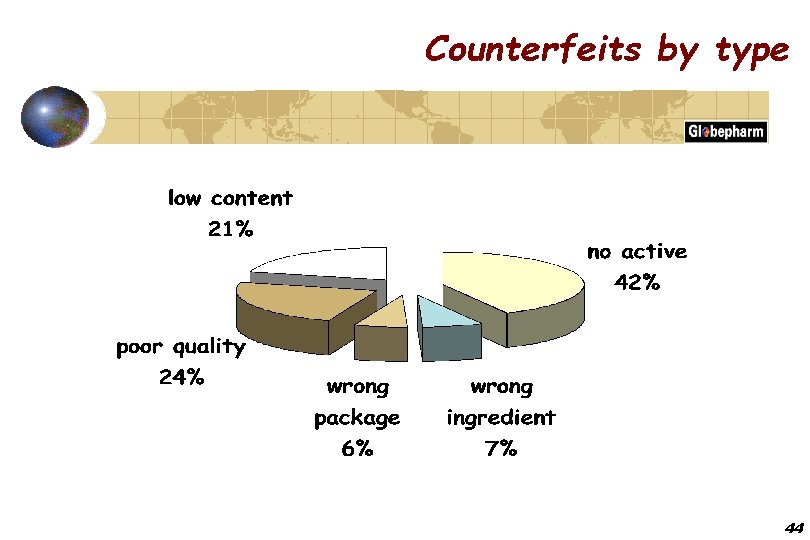
Counterfeits by type 44
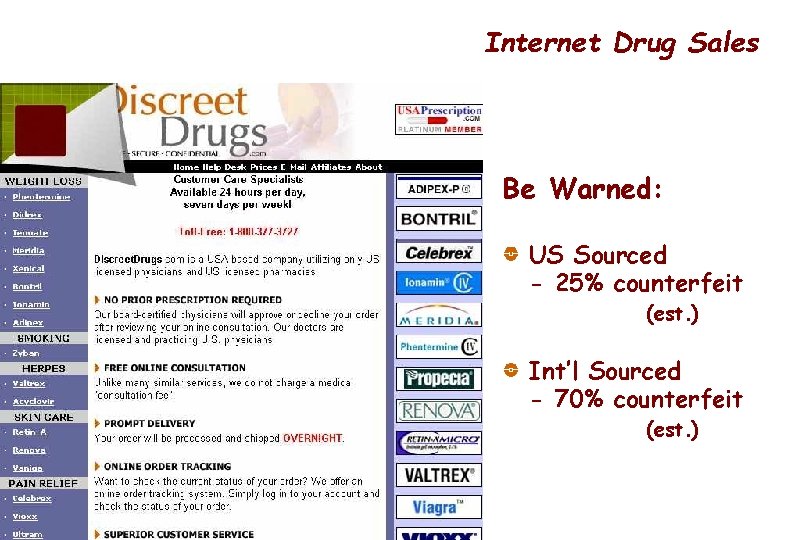
Internet Drug Sales Be Warned: US Sourced - 25% counterfeit (est. ) Int’l Sourced - 70% counterfeit (est. ) 45
46
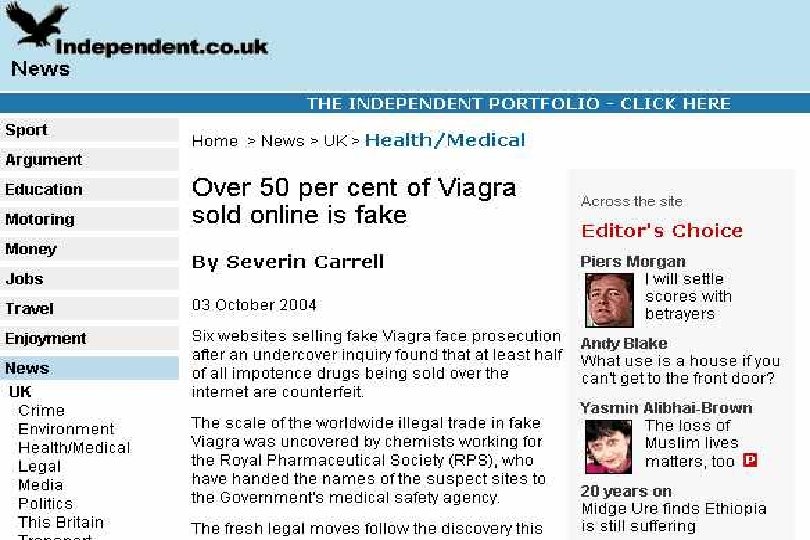
47

48
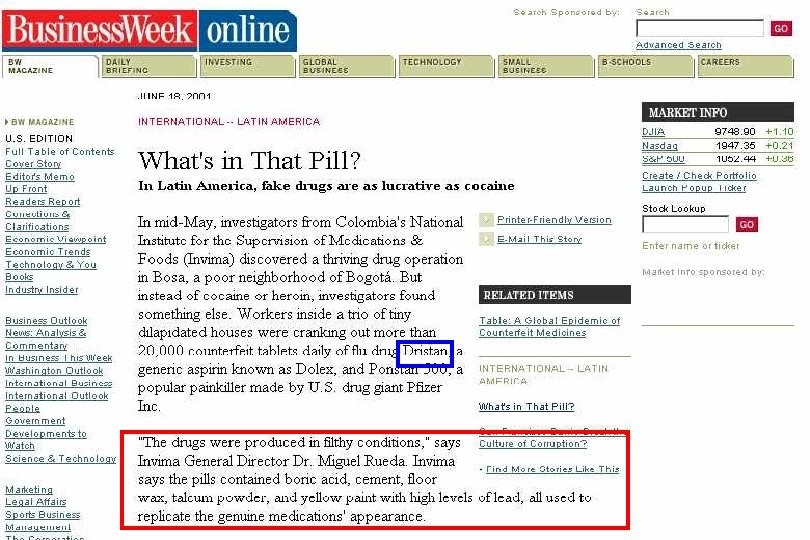
49

50

Slick Packaging 51

Fake Drugs – Fake Companies 52

Counterfeit Documentation 53
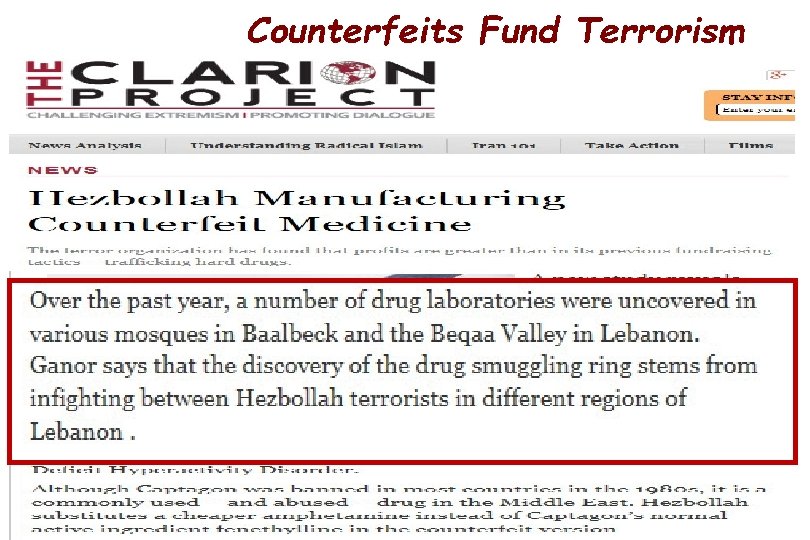
Counterfeits Fund Terrorism

55

Street Market Sales - Nigeria 56

EU – Falsified Medicines Directive July 2011 Definition – Falsified Medicinal Product Any medicinal product with a false representation of: a. its identity, including its packaging and labelling, its name or its composition as regards any of the ingredients including excipients and the strength of those ingredients; b. its source, including its manufacturer, its country of manufacturing, its country of origin or its marketing authorisation holder; or c. its history, including the records and documents relating to the distribution channels used. This definition does not include unintentional quality defects and is without prejudice to infringements of intellectual property rights.
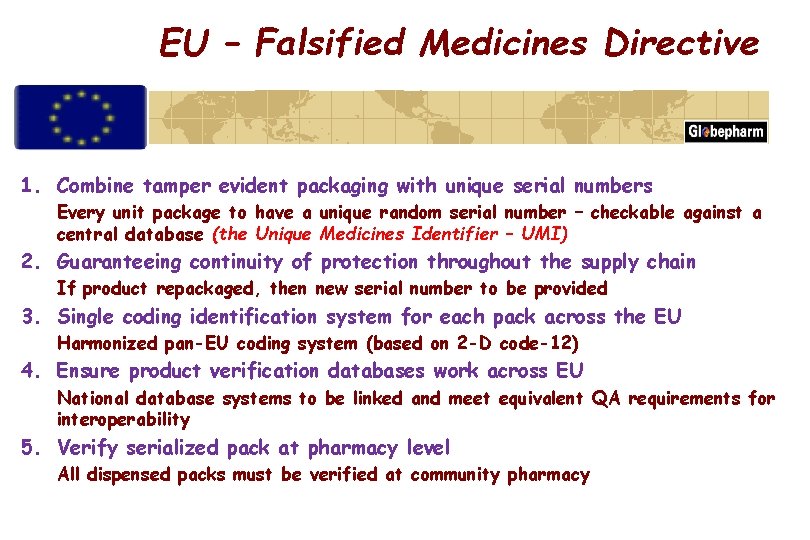
EU – Falsified Medicines Directive 1. Combine tamper evident packaging with unique serial numbers Every unit package to have a unique random serial number – checkable against a central database (the Unique Medicines Identifier – UMI) 2. Guaranteeing continuity of protection throughout the supply chain If product repackaged, then new serial number to be provided 3. Single coding identification system for each pack across the EU Harmonized pan-EU coding system (based on 2 -D code-12) 4. Ensure product verification databases work across EU National database systems to be linked and meet equivalent QA requirements for interoperability 5. Verify serialized pack at pharmacy level All dispensed packs must be verified at community pharmacy
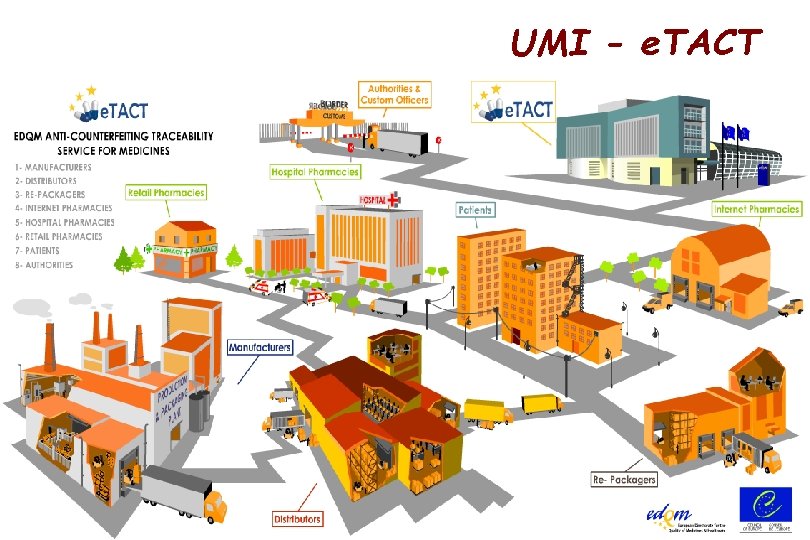
UMI - e. TACT

Anti-Counterfeit Technologies USA – Pharmaceutical Distribution Security Alliance (PDSA) Pharmaceutical manufacturers, wholesalers, distributors and retailers Rx. TEC 60

Tracking and Tracing

Anti-Counterfeit Technologies Overt Techniques Raised printing Color shifting inks Watermarks Holograms RFID Sproxil® Label Covert Techniques Microtext Microthreads Nanoencryption Chemical Taggants UV inks Forensic Techniques Extractables + leachables Physico-chemical identifiers – inks, pigments, flavors, molecular taggants

Counterfeiting Medicines Is Not New! Want To See A Great Classic Movie? Vienna 1945

Questions ?
Temperature Profiling Maintaining the Cold Chain

Key Topics: 21 CFR 211. 142 • Store in appropriate conditions WHO QAS/04. 068 • GDP applicable to all pharmaceutical products • Temperature mapping of vehicles Health Canada Guide 0069 • Supply-chain rules • Temperature monitoring of distribution chain Cold Chain Protection Brazil For import release, all incoming biological products must have temperature-monitoring records showing proper storage temperatures. EU Ensure storage conditions at all times Controlled temperature products (15 -25 °C) should be transported by appropriate means Temperature review is part of QP release Integrated Approach to temperature protection Stabilize the ambient temperature profile through procedure Contingency Response through near-real-time monitoring In-transit infrastructure / procedure Temperature records Easily available upon arrival at port of entry for customs/regulatory release Balance Product stability, Process Control, and Package

Temperature Profiling - Warehousing 38 o. C 26 o. C Controlled Room Temperature — a working environment of 20 o. C to 25 o. C; that results in a mean kinetic temperature calculated to be not more than 25 o. C; and that allows for excursions between 15 o. C and 30 o. C experienced in pharmacies, hospitals, and warehouses. Transient spikes up to 40 o. C are permitted that do not exceed 24 hours. 23 o. C
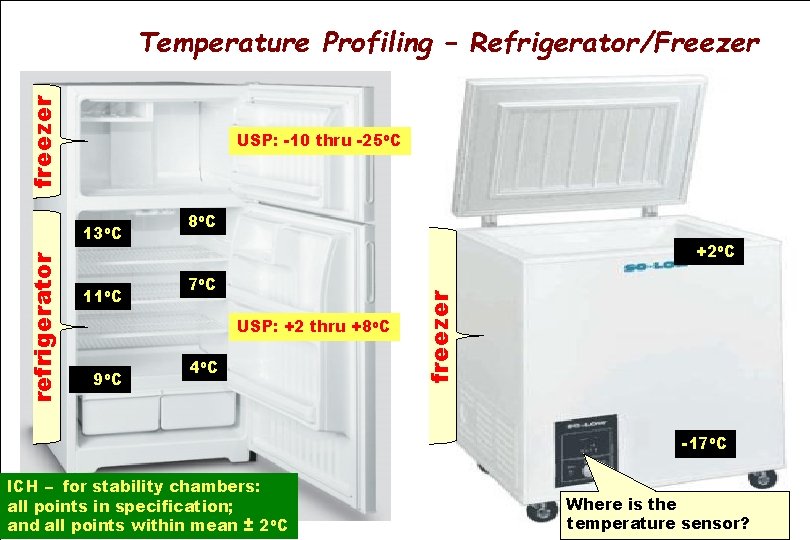
freezer Temperature Profiling – Refrigerator/Freezer USP: -10 thru -25 o. C 11 o. C 8 o. C +2 o. C 7 o. C USP: +2 thru +8 o. C 9 o. C 4 o. C freezer refrigerator 13 o. C -17 o. C ICH – for stability chambers: all points in specification; and all points within mean ± 2 o. C Where is the temperature sensor?
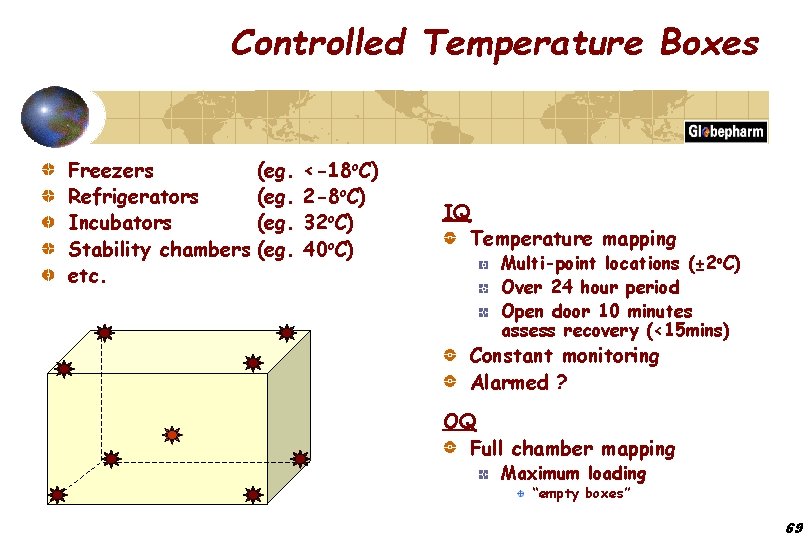
Controlled Temperature Boxes Freezers Refrigerators Incubators Stability chambers etc. (eg. <-18 o. C) 2 -8 o. C) 32 o. C) 40 o. C) IQ Temperature mapping Multi-point locations (± 2 o. C) Over 24 hour period Open door 10 minutes assess recovery (<15 mins) Constant monitoring Alarmed ? OQ Full chamber mapping Maximum loading “empty boxes” 69

Global Shipping Concerns 70
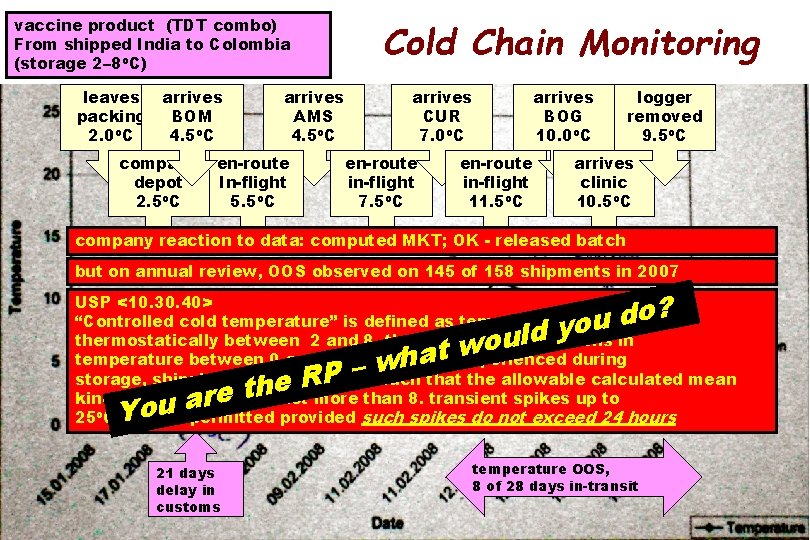
vaccine product (TDT combo) From shipped India to Colombia (storage 2– 8 o. C) leaves packing 2. 0 o. C arrives BOM 4. 5 o. C company depot 2. 5 o. C arrives AMS 4. 5 o. C en-route In-flight 5. 5 o. C Cold Chain Monitoring arrives CUR 7. 0 o. C en-route in-flight 7. 5 o. C en-route in-flight 11. 5 o. C arrives BOG 10. 0 o. C logger removed 9. 5 o. C arrives clinic 10. 5 o. C company reaction to data: computed MKT; OK - released batch but on annual review, OOS observed on 145 of 158 shipments in 2007 ? o d u o y d l u o w t ha w – P R e th e r a You USP <10. 30. 40> “Controlled cold temperature” is defined as temperature maintained 8 o. C thermostatically between 2 and 8, that allows for excursions in temperature between 0 and 15 that may be experienced during storage, shipping, and distribution such that the allowable calculated mean o. C kinetic temperature is not more than 8. transient spikes up to 2 25 o. C may be permitted provided such spikes do not exceed 24 hours 21 days delay in customs temperature OOS, 8 of 28 days in-transit 71
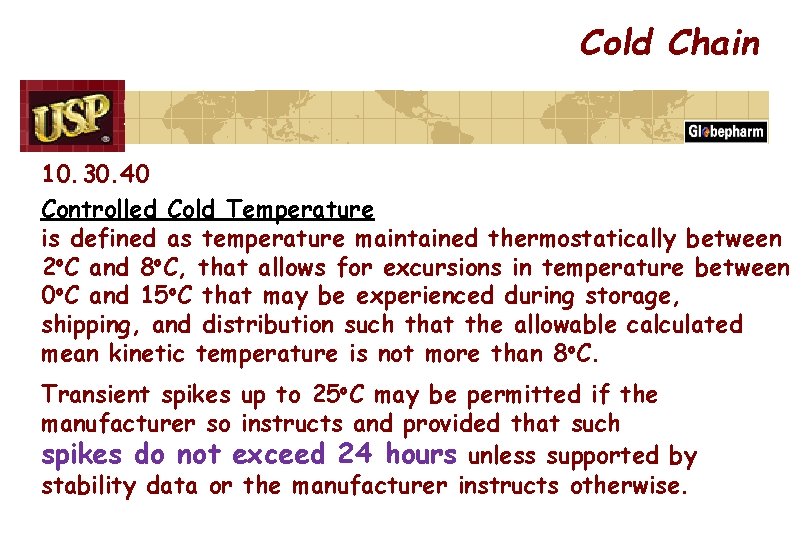
Cold Chain 10. 30. 40 Controlled Cold Temperature is defined as temperature maintained thermostatically between 2 o. C and 8 o. C, that allows for excursions in temperature between 0 o. C and 15 o. C that may be experienced during storage, shipping, and distribution such that the allowable calculated mean kinetic temperature is not more than 8 o. C. Transient spikes up to 25 o. C may be permitted if the manufacturer so instructs and provided that such spikes do not exceed 24 hours unless supported by stability data or the manufacturer instructs otherwise.

Riyadh, Saudi Arabia – Daytime High (May 2013) +117 o. F (+47 o. C) Ulan Bator, Mongolia – Daytime Low (Dec 2012) – 42 o. F (– 41 o. C) 60% of total air shipment time is spent at the airport 54% of temperature excursions occur when drug product is in airline possession 73

Passive Packaging Configurations 74

Active Packaging Configurations Refrigeration Unit (electric/battery power) 75
Cold Chain Monitors 76

Data Loggers 77
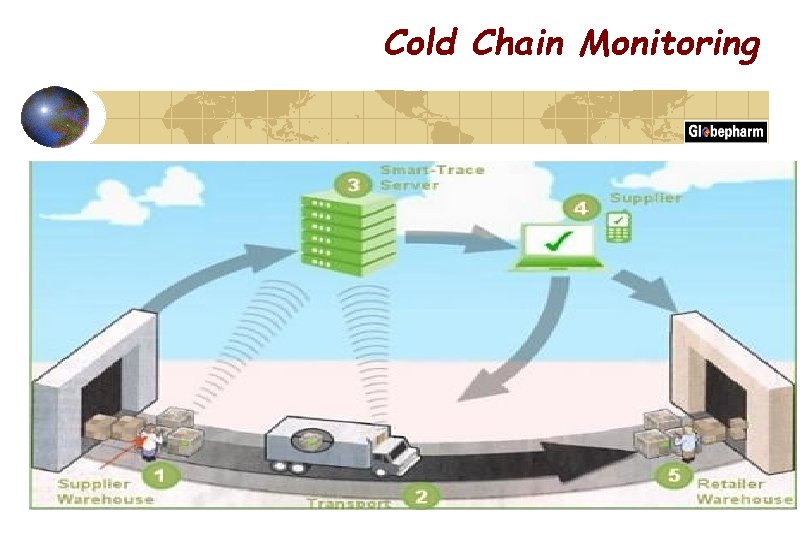
Cold Chain Monitoring

Product Integrity Theft / Tampering
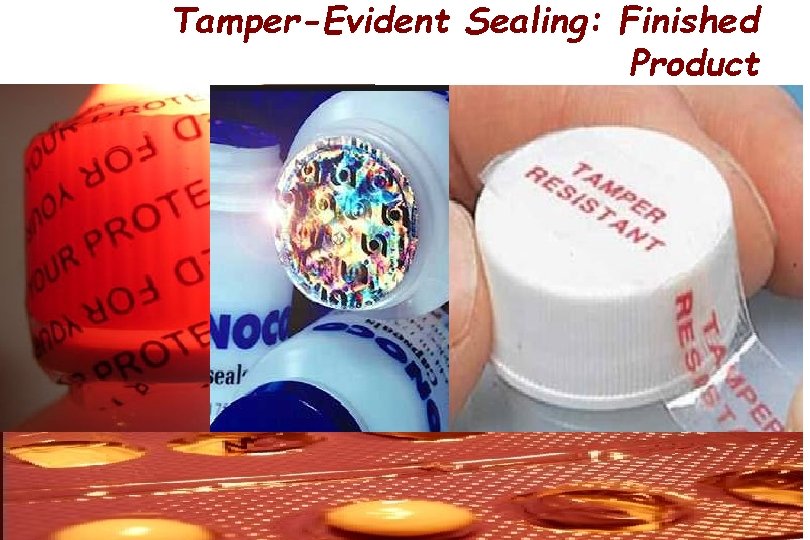
Tamper-Evident Sealing: Finished Product

Tamper-Evident Sealing: Raw Materials

Product Integrity Sanitation

Sanitation

Mongoose vs. Snake 84

Product Integrity Stock Rotation

FIFO – LIFO/FEFO - FISH FIFO first-in-first-out LIFO last-in-first-out FEFO first-expired-first-out FISH first-in-still-here Not in a GMP World

Product Integrity Documentation Record Keeping

GDP Record Keeping 14. DOCUMENTATION 14. 1 SOPs and records to document all activities relating to the distribution of pharmaceutical products, including all applicable receipts and issues. 14. 2 Records should be kept by distributors of all pharmaceutical products received which contain at least the following information: date, name of the pharmaceutical product, quantity received, or supplied, name and address of the supplier. 14. 3 SOPs for the preparation, review, approval, use of and control of changes to all documents relating to the distribution process. Procedures must be in place for both internally generated documents and those from external sources. . 14. 4 Documents, and SOPs relating to any activity that could have an impact on the quality of pharmaceutical products, should be designed, completed, reviewed and distributed with care. 14. 5 The title, nature and purpose of each document should be clearly stated. The contents of documents should be clear and unambiguous. Documents should be laid out in an orderly fashion and be easy to check. 14. 6 All documents should be completed, approved, signed (as required) and dated by an appropriate authorized person(s) and should not be changed without the necessary authorization.

GDP Record Keeping 14. 7 The nature, content and retention of documentation relating to the distribution of pharmaceutical products and any investigations conducted and action taken, should comply with national legislative requirements. Where such requirements are not in place, the documents should be retained for at least one year after the expiry date of the product. 14. 8 The distributor must establish and maintain procedures for the identification, collection, indexing, retrieval, storage, maintenance, disposal of and access to all applicable documentation. 14. 9 All records must be readily retrievable, and be stored and retained using facilities that are safeguarded against unauthorized modification, damage, deterioration and/or loss of documentation. 14. 10 Documents should be reviewed regularly and kept up to date. When a document has been revised, a system should exist to prevent inadvertent use of the superseded version. 14. 11 Mechanisms should exist to allow for transfer of information, including quality or regulatory information, between a manufacturer and a customer, as well as the transfer of information to the relevant regulatory authority as required. 14. 12 Records relating to storage of pharmaceutical products should be kept and be readily available upon request in accordance with the WHO guidelines on good storage practice (1).

GDP Record Keeping 14. 13 Permanent records, written or electronic, should exist for each stored product indicating recommended storage conditions, any precautions to be observed and retest dates. Pharmacopoeial requirements and current national regulations concerning labels and containers should be respected at all times. 14 Procedures should be in place for temperature mapping, security services to prevent theft or tampering with goods at the storage facilities, destruction of unsaleable/unusable stocks and on retention of the records. 14. 15 Where the records are generated and kept in electronic form, backups should be maintained to prevent any accidental data loss.

Questions ?

STAY IN TOUCH Michael H. Anisfeld Globepharm Consulting manisfeld@globepharm. org

Good Distribution Practices obrigado!
- Slides: 93