Goal Directed Fluid Therapy An Essential Component of



























- Slides: 49

Goal Directed Fluid Therapy: An Essential Component of ERAS D. John Doyle MD Ph. D Chief, Department of General Anesthesia Cleveland Clinic Abu Dhabi No Conflicts of Interest No Financial Disclosures

Some Take Home Points • The classical approach to fluid administration during surgery is flawed. • Third-spacing is a myth. • Fluid boluses may be harmful. • CVP measurements are not as useful as we once thought and high CVP levels may be harmful. • Earlier recommendations for fluid management in sepsis seem to be mistaken. • Individualized goal-directed fluid therapy (GDFT) is recommended, which may include zero-balance fluid management.

Let’s Start with a Cautionary Tale

2011 - A Cautionary Tale Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011 Jun 30; 364(26): 2483 -95. “Fluid boluses significantly increased 48 -hour mortality in critically ill children with impaired perfusion in these resource-limited settings in Africa. ”

FEAST Trial “Fluid Expansion As Supportive Therapy” Patients were children with infectious shock (e. g. , from malaria); median age 24 months Inclusion criteria: • Severe febrile illness AND • Impaired consciousness or respiratory distress AND • Impaired perfusion (including capillary refill time, tachycardia, thready radial pulse or lower limb temperature gradient) In stratum A each child was randomised to either: • Bolus 0. 9% Saline (20 ml/kg over one hour) OR • Bolus 5% Albumin (20 ml/kg over one hour) OR • Maintenance fluids (2. 5— 4 ml/kg/hour)

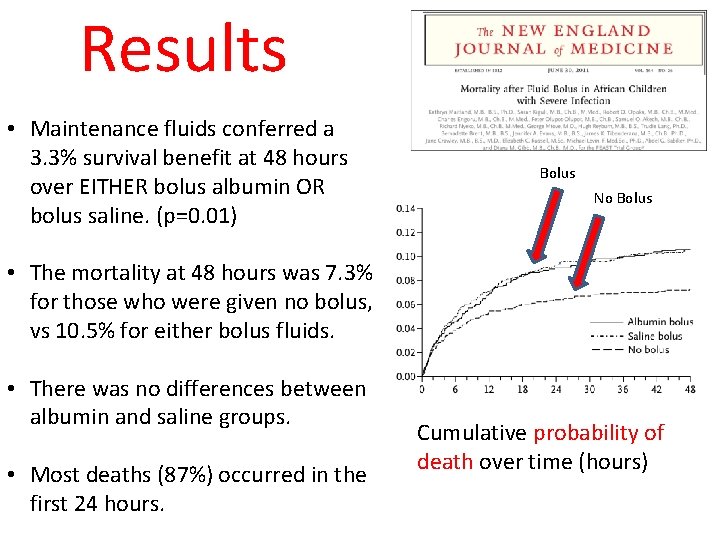
Results • Maintenance fluids conferred a 3. 3% survival benefit at 48 hours over EITHER bolus albumin OR bolus saline. (p=0. 01) Bolus No Bolus • The mortality at 48 hours was 7. 3% for those who were given no bolus, vs 10. 5% for either bolus fluids. • There was no differences between albumin and saline groups. • Most deaths (87%) occurred in the first 24 hours. Cumulative probability of death over time (hours)

Lessons Learned This was a landmark study investigating the effects of fluid boluses in the resuscitation of febrile children with poor perfusion. The unexpected results require that we reassess our assumptions about fluid resuscitation.

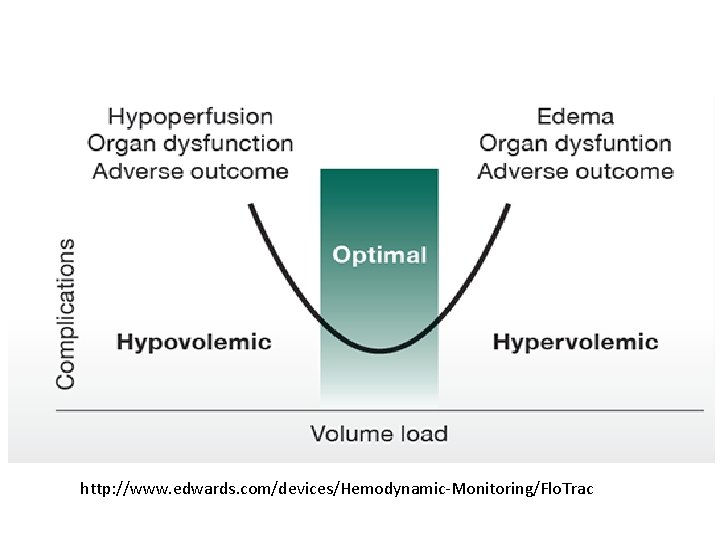
http: //www. edwards. com/devices/Hemodynamic-Monitoring/Flo. Trac

Three schools of thought for fluid therapy during major surgery The classical approach - results in a postoperative weight increase of 3– 6 kg from replacement of fluid losses, including the so-called third-space losses. Still taught in some anesthesia textbooks. The fluid bolus approach - where fluid boluses are given to reach near-maximal stroke volume, (e. g. , as measured via esophageal Doppler), a defined CVP range, a defined Scv. O 2 range, etc. The restricted approach - where all measured fluid losses are replaced with a goal of zero fluid balance and without the replacement of third-space losses. This approach is based on the hypothesis that excess fluid causes interstitial edema harmful for tissue healing and cardiac and pulmonary function. Modified from: Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt SÅ, Hansen B, Møller DR, Lundbech LB, Andersen N, Berg V, Thomassen N, Andersen ST, Simonsen L. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: nearmaximal stroke volume or zero fluid balance? Br J Anaesth. 2012 Aug; 109(2): 191 -9.

Which approach is best?

What about the classical approach?

The Problem with the Classical Approach: Third-Spacing is a Myth

Third-Spacing is a Myth “In summary, a classic third space was never localized and only “quantified” with one specific method using certain conditions regarding sampling and equilibration times, implying serious concerns and weaknesses. All other methods using various tracers, multiple sampling techniques, longer equilibration times, or analysis of kinetics contradict the existence of a fluid-consuming third space. Taking all this into account, we have to conclude that a classic third space per se quantitatively does not exist. It is currently not more than an ill-defined compartment thought to reflect an otherwise unexplainable perioperative fluid shift. Therefore, we suggest abolishing this mystery and sticking to the given facts: Fluid is perioperatively shifted within the functional extracellular compartment, from the intravascular toward the interstitial space” Chappel D et. al. Anesthesiology 109, 723: 2008

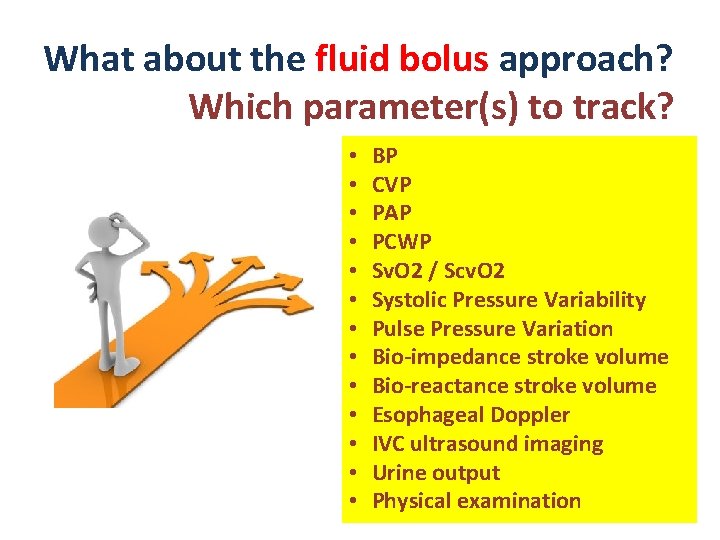
What about the fluid bolus approach? Which parameter(s) to track? • • • • BP CVP PAP PCWP Sv. O 2 / Scv. O 2 Systolic Pressure Variability Pulse Pressure Variation Bio-impedance stroke volume Bio-reactance stroke volume Esophageal Doppler IVC ultrasound imaging Urine output Physical examination

Some More Disappointing Studies

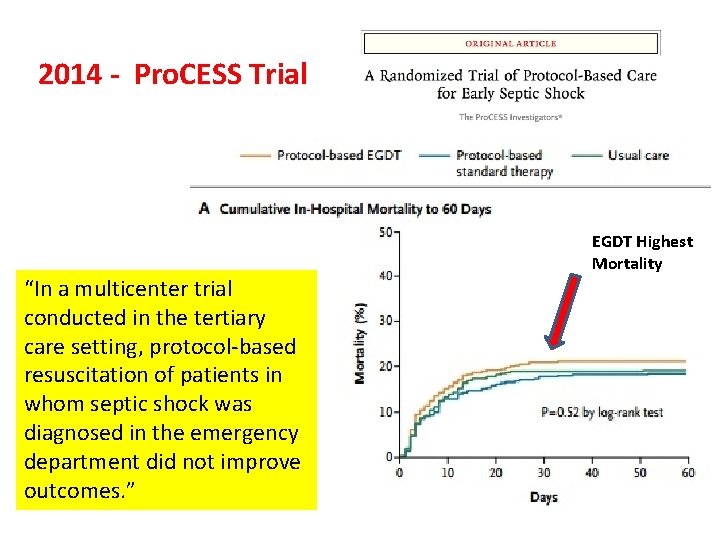
2014 - Pro. CESS Trial Pro. CESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, Lo. Vecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014 May 1; 370(18): 1683 -93. “In a multicenter trial conducted in the tertiary care setting, protocol-based resuscitation of patients in whom septic shock was diagnosed in the emergency department did not improve outcomes. ”

2014 - Pro. CESS Trial NEngl J Med. 2014 May 1; 370(18): 1683 -93 INTERVENTIONS/ COMPARISONS • Protocol-based standard therapy n=446 • Early goal directed therapy (EGDT) n=439 (as per Rivers protocol) • Usual care n=458 (at the discretion of the treating physician) OUTCOMES • Primary outcome: No difference in in-hospital death at 60 days: EGDT 21%, Protocol 18. 2%, Usual care 18. 9% • Secondary outcome: More ICU admissions in the EGDT group: EGDT 91. 3%, Protocol 85. 4%, Usual care 86. 2%

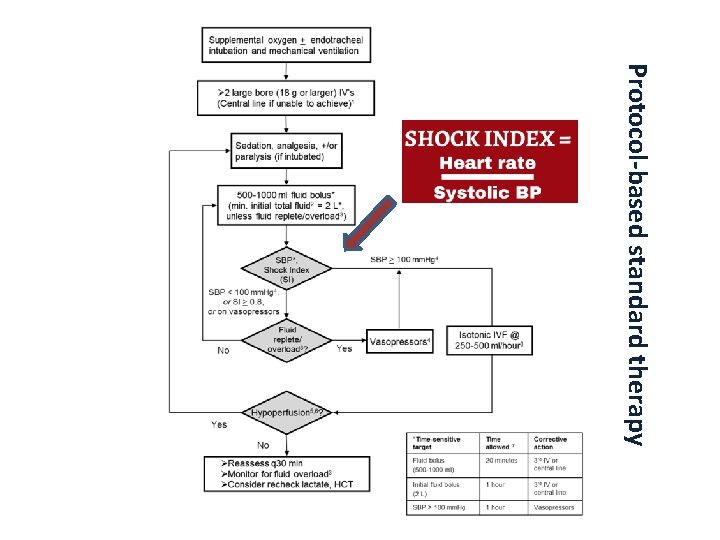
Protocol-based standard therapy

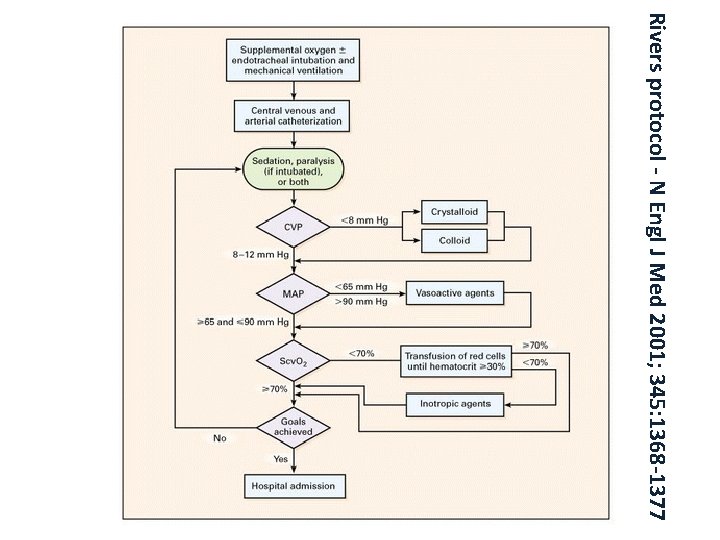
Rivers protocol - N Engl J Med 2001; 345: 1368 -1377

2014 - Pro. CESS Trial “In a multicenter trial conducted in the tertiary care setting, protocol-based resuscitation of patients in whom septic shock was diagnosed in the emergency department did not improve outcomes. ” EGDT Highest Mortality

2014 – ARISE Trial N Engl J Med 2014; 371: 1496 -506. Early goal-directed therapy (EGDT) has been endorsed to decrease mortality among patients presenting with septic shock. However, in critically ill patients presenting to the emergency department with early septic shock, EGDT based on continuous Scv. O 2 (central venous oxygen saturation) measurements did not reduce all-cause mortality at 90 days. Incorporating EGDT based on Scv. O 2 into guidelines is questionable.

2014 The FENICE study Evaluated approaches to fluid resuscitation in 46 countries and concluded that the “current practice and evaluation of fluid management in critically ill patients seems to be arbitrary… is not evidence-based and could be harmful. ” Cecconi M, Hofer C, Teboul JL, et al; FENICE Investigators and the ESICM Trial Group: Fluid challenges in intensive care: The FENICE study: A global inception cohort study. Intensive Care Med 2015; 41: 1529– 1537

Time for a re-examination

2015 Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS). Can J Anaesth. 2015 Feb; 62(2): 158 -68. Optimal perioperative fluid management is an important component of Enhanced Recovery After Surgery (ERAS) pathways. Fluid management within ERAS should be viewed as a continuum through the preoperative, intraoperative, and postoperative phases. Each phase is important for improving patient outcomes, and suboptimal care in one phase can undermine best practice within the rest of the ERAS pathway. The goal of preoperative fluid management is for the patient to arrive in the operating room in a hydrated and euvolemic state. To achieve this, prolonged fasting is not recommended, and routine mechanical bowel preparation should be avoided. Patients should be encouraged to ingest a clear carbohydrate drink two to three hours before surgery. The goals of intraoperative fluid management are to maintain central euvolemia and to avoid excess salt and water. To achieve this, patients undergoing surgery within an enhanced recovery protocol should have an individualized fluid management plan. As part of this plan, excess crystalloid should be avoided in all patients. For low-risk patients undergoing low-risk surgery, a “zero-balance” approach might be sufficient. In addition, for most patients undergoing major surgery, individualized goaldirected fluid therapy (GDFT) is recommended. Ultimately, however, the additional benefit of GDFT should be determined based on surgical and patient risk factors. Postoperatively, once fluid intake is established, intravenous fluid administration can be discontinued and restarted only if clinically indicated. In the absence of other concerns, detrimental postoperative fluid overload is not justified and “permissive oliguria” could be tolerated.

“Several recent studies performed to test the effectiveness of GDFT within an ERAS protocol have failed to find the same benefit on postoperative outcomes as that found in earlier studies. Perhaps this is not surprising, as significant improvement in perioperative fluid management within an ERAS protocol, particularly in the past 15 years, has facilitated significant improvement in the quality of care in control groups of fluid management studies. ” Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS). Can J Anaesth. 2015 Feb; 62(2): 158 -68.

2016 'Enhanced recovery after surgery' (ERAS) protocols implement a series of peri-operative interventions intended to improve recovery after major operations, one aspect of which is fluid management. The pre-operative goal is to prepare a hydrated, euvolaemic patient by avoiding routine mechanical bowel preparation and by encouraging patients to drink clear liquids up to two hours before induction of anaesthesia. The intraoperative goal is to achieve a 'zero' fluid balance at the end of uncomplicated surgery: goal-directed fluid therapy is recommended for poorly prepared or sick patients or those undergoing more complex surgery. The postoperative goal is eating and drinking without intravenous fluid infusions. Postoperative oliguria should be expected and accepted, as urine output does not indicate overall fluid status. Gupta R, Gan TJ. Peri-operative fluid management to enhance recovery. Anaesthesia. 2016 Jan; 71 Suppl 1: 40 -5

Fluid Therapy for ERAS • Pre-operative goals • Intra-operative goals • Postoperative goals

Fluid Therapy for ERAS “The pre-operative goal is to prepare a hydrated, euvolaemic patient by avoiding routine mechanical bowel preparation and by encouraging patients to drink clear liquids up to two hours before induction of anaesthesia. ” Gupta R, Gan TJ. Peri-operative fluid management to enhance recovery. Anaesthesia. 2016 Jan; 71 Suppl 1: 40 -5.

Fluid Therapy for ERAS “The intra-operative goal is to achieve a 'zero' fluid balance at the end of uncomplicated surgery; goaldirected fluid therapy is recommended for poorly prepared or sick patients or those undergoing more complex surgery. ” Gupta R, Gan TJ. Peri-operative fluid management to enhance recovery. Anaesthesia. 2016 Jan; 71 Suppl 1: 40 -5.

Fluid Therapy for ERAS “The postoperative goal is eating and drinking without intravenous fluid infusions. Postoperative oliguria should be expected and accepted, as urine output does not indicate overall fluid status. ” Gupta R, Gan TJ. Peri-operative fluid management to enhance recovery. Anaesthesia. 2016 Jan; 71 Suppl 1: 40 -5.

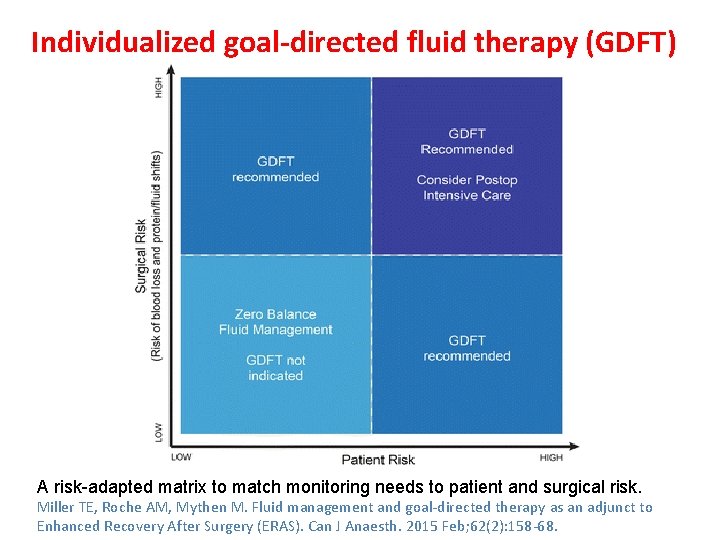
Individualized goal-directed fluid therapy (GDFT) A risk-adapted matrix to match monitoring needs to patient and surgical risk. Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS). Can J Anaesth. 2015 Feb; 62(2): 158 -68.

Perils of Aggressive Fluid Resuscitation New evidence suggests that aggressive fluid resuscitation leads to severe tissue edema that compromises organ function and leads to increased morbidity and mortality Marik PE: Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care 2014; 4: 21 Kelm DJ, Perrin JT, Cartin-Ceba R, et al: Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock 2015; 43: 68– 73

Zero-Balance Fluid Management

Fluid Responsiveness and the Six Guiding Principles of Fluid Resuscitation Paul E. Marik, Crit Care Med 2016

Principle 1 Fluid boluses are most frequently given to hypotensive patients; however, only patients who are fluid responsive should be resuscitated with fluid boluses.

Principle 1 The only reason to give a patient a fluid challenge is to increase the stroke volume (SV); if this does not happen, fluid administration serves no purpose and may be harmful. A patient is considered to be fluid responsive if the SV increases by at least 10% following a fluid challenge (usually 500 cc of crystalloid) Marik PE, Monnet X, Teboul JL: Hemodynamic parameters to guide fluid therapy. Ann Intensive Care 2011; 1: 1

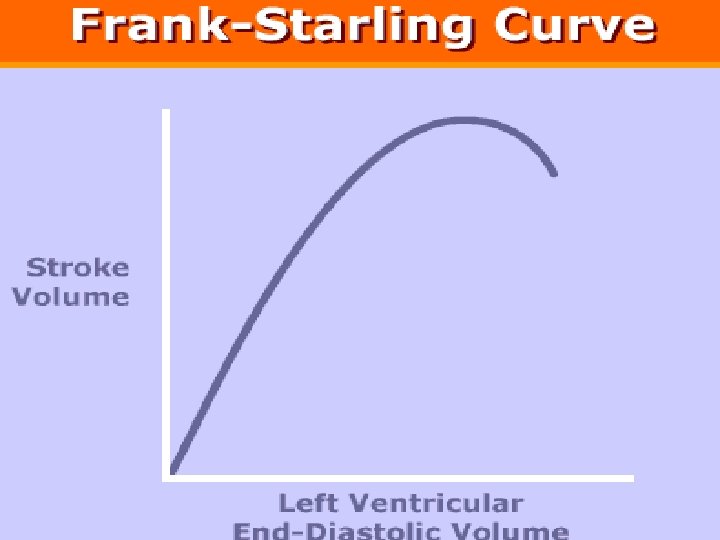
Fluid Administration to Increase SV Fluid administration will increase SV only if two conditions are met: [1] The fluid bolus increases the “stressed blood volume” [2] Both ventricles are functioning on the ascending limb of the Frank-Starling curve. Marik PE. The physiology of volume resuscitation. Curr Anesthesiol Rep 2014; 4: 353– 359

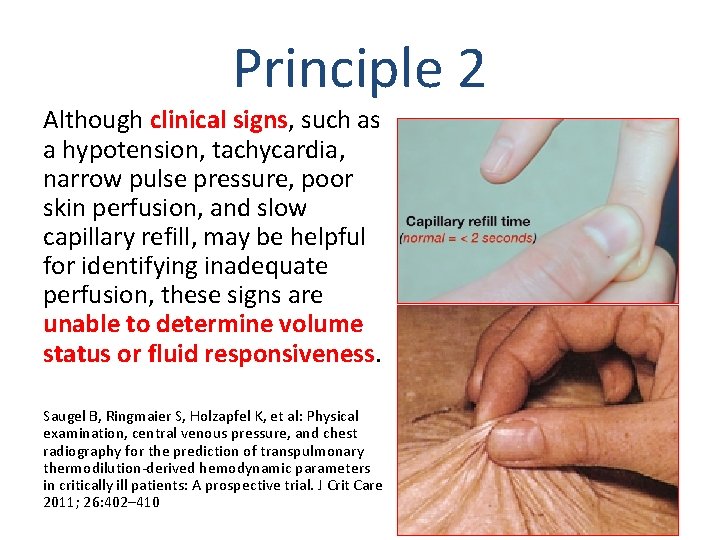
Principle 2 Although clinical signs, such as a hypotension, tachycardia, narrow pulse pressure, poor skin perfusion, and slow capillary refill, may be helpful for identifying inadequate perfusion, these signs are unable to determine volume status or fluid responsiveness. Saugel B, Ringmaier S, Holzapfel K, et al: Physical examination, central venous pressure, and chest radiography for the prediction of transpulmonary thermodilution-derived hemodynamic parameters in critically ill patients: A prospective trial. J Crit Care 2011; 26: 402– 410

Principle 2 Additionally…. The CVP or change in CVP following a fluid challenge is not accurate in predicting fluid responsiveness (Crit Care Med 2013; 41: 1774– 1781). The change in the mean arterial pressure (MAP) following a fluid bolus is also poorly predictive of fluid responsiveness (Intensive Care Med 2015; 41: 1247– 1255). Ultrasonography of the IVC and its respiratory variation are no more predictive than the CVP for assessing fluid responsiveness (Emerg Med Australas 2012; 24: 534– 539).

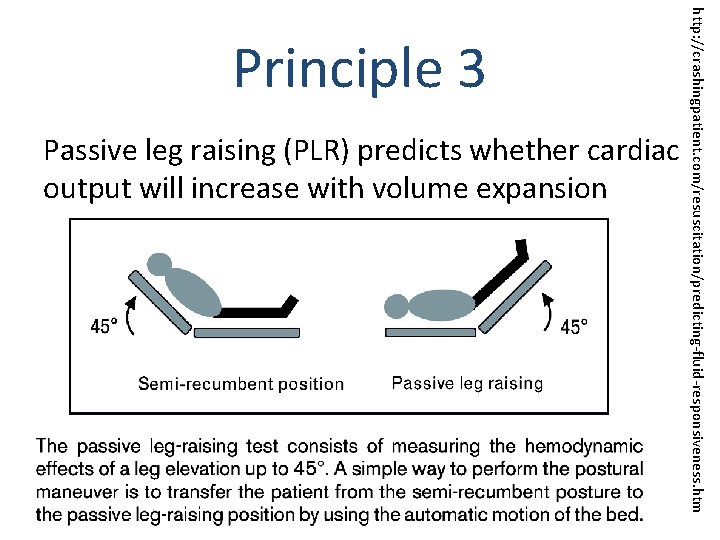
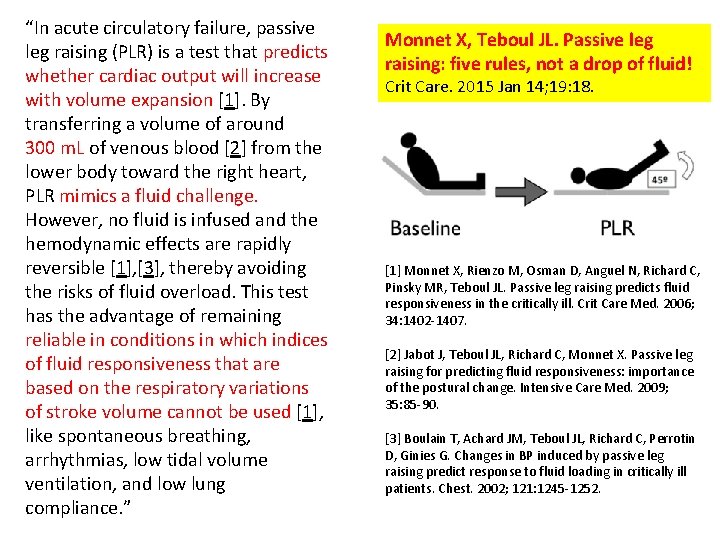
Passive leg raising (PLR) predicts whether cardiac output will increase with volume expansion http: //crashingpatient. com/resuscitation/predicting-fluid-responsiveness. htm Principle 3

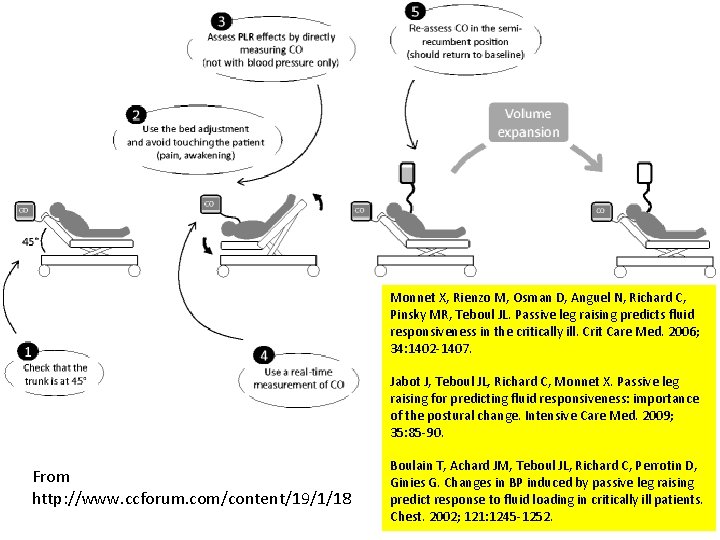
“In acute circulatory failure, passive leg raising (PLR) is a test that predicts whether cardiac output will increase with volume expansion [1]. By transferring a volume of around 300 m. L of venous blood [2] from the lower body toward the right heart, PLR mimics a fluid challenge. However, no fluid is infused and the hemodynamic effects are rapidly reversible [1], [3], thereby avoiding the risks of fluid overload. This test has the advantage of remaining reliable in conditions in which indices of fluid responsiveness that are based on the respiratory variations of stroke volume cannot be used [1], like spontaneous breathing, arrhythmias, low tidal volume ventilation, and low lung compliance. ” Monnet X, Teboul JL. Passive leg raising: five rules, not a drop of fluid! Crit Care. 2015 Jan 14; 19: 18. [1] Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006; 34: 1402 -1407. [2] Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med. 2009; 35: 85 -90. [3] Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest. 2002; 121: 1245 -1252.

Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006; 34: 1402 -1407. Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med. 2009; 35: 85 -90. From http: //www. ccforum. com/content/19/1/18 Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest. 2002; 121: 1245 -1252.

Noninvasive Real-Time Cardiac Output

Principle 4 The hemodynamic response to a fluid challenge is usually small and short lived. Example study: Nunes et al. evaluated the effect duration of a fluid bolus in patients in shock. 65% of patients were fluid responders whose cardiac index increased by 25% at the end of the 30 min infusion. However, the cardiac index had returned to baseline 30 minutes after the end of the infusion. (Ann Intensive Care 2014; 4: 25)

Principle 5 Most healthy humans function on the ascending limb of the Frank-Starling curve; they have preload reserve and do not require fluid. Similarly, most critically ill and injured patients and patients undergoing surgery do not need to be pushed to the top of their Frank-Starling curve. Patients should only receive a fluid bolus if they are preload responsive and expected to benefit from the fluid.


Principle 6 High CVP levels are bad! “There are now compelling data that the primary hemodynamic goal in critically ill and injured patients and those undergoing surgery is an MAP of greater than 65 mm Hg and a CVP of less than 8 mm Hg. Remarkably, this CVP target contradicts current guidelines that recommend targeting a CVP of greater than 8 mm Hg. ”

• The classical approach to fluid administration during surgery is Conclusions flawed. • Third-spacing is a myth. • Fluid boluses may be harmful. • CVP measurements are not as useful as we once thought. • High CVP levels may be harmful. • Earlier recommendations for fluid management in sepsis seem to be mistaken. • Individualized goal-directed fluid therapy (GDFT) is recommended, which may include zero-balance fluid management. Almost everything you learned about perioperative fluid therapy is now suspect!

The End