Glycolysis Phase 1 and 2 Phase 1 Sugar








- Slides: 34

Glycolysis: Phase 1 and 2 § Phase 1: Sugar activation § two ATP molecules used to activate glucose into: § § Phase 2: Sugar cleavage § Fructose-1, 6 -bisphosphate is cleaved into: § § fructose-1, 6 -diphosphate two 3 -carbon isomers Phase 3: Oxidation and ATP formation § 3 -carbon sugars are oxidized (reducing NAD+) § ATP is formed by substrate-level phosphorylation Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

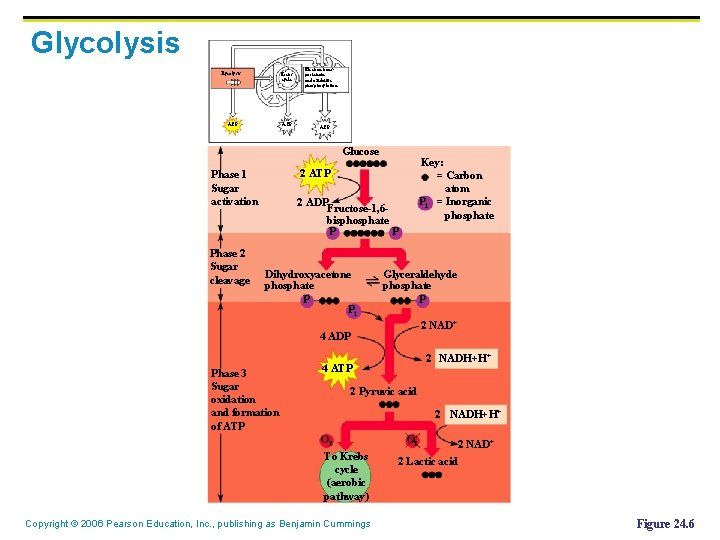
Glycolysis Krebs cycle ATP Electron transport chain and oxidative phosphorylation ATP Glucose Phase 2 Sugar cleavage Key: = Carbon atom Pi = Inorganic phosphate 2 ATP Phase 1 Sugar activation 2 ADP Fructose-1, 6 bisphosphate P P Dihydroxyacetone phosphate P Pi Glyceraldehyde phosphate P 2 NAD+ 4 ADP Phase 3 Sugar oxidation and formation of ATP 2 NADH+H+ 4 ATP 2 Pyruvic acid 2 NADH+H+ O 2 To Krebs cycle (aerobic pathway) Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings O 2 2 NAD+ 2 Lactic acid Figure 24. 6

Glycolysis: Phase 3 § The final products of glycolysis: § Two pyruvic acid molecules § Two NADH + H+ molecules (reduced NAD+) § A net gain of two ATP molecules (4 ATP- 2 ATP) Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

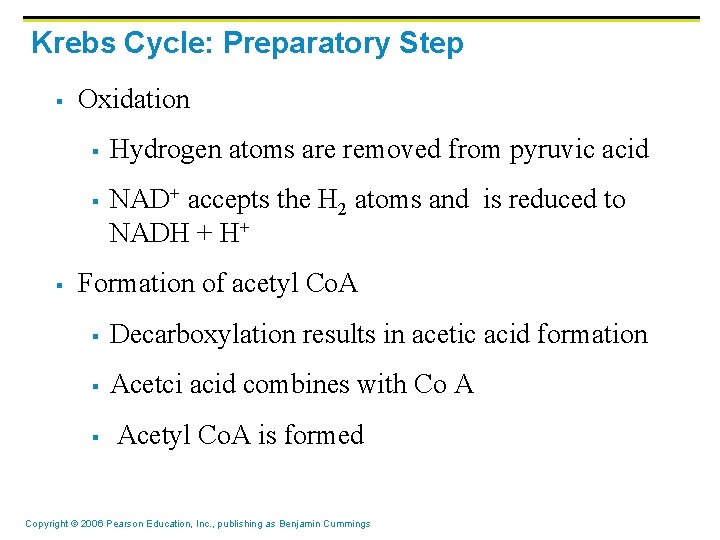
Krebs Cycle: Preparatory Step § § § Occurs inside the mitochondria in the mitochondrial matrix Fueled by: § Pyruvic acid (carbohydrates) § Fatty acids (lipids) Pyruvic acid is converted to acetyl Co. A in three main steps: § Decarboxylation § Carbon is removed from pyruvic acid § Carbon dioxide is released Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Krebs Cycle: Preparatory Step § Oxidation § § § Hydrogen atoms are removed from pyruvic acid NAD+ accepts the H 2 atoms and is reduced to NADH + H+ Formation of acetyl Co. A § Decarboxylation results in acetic acid formation § Acetci acid combines with Co A § Acetyl Co. A is formed Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

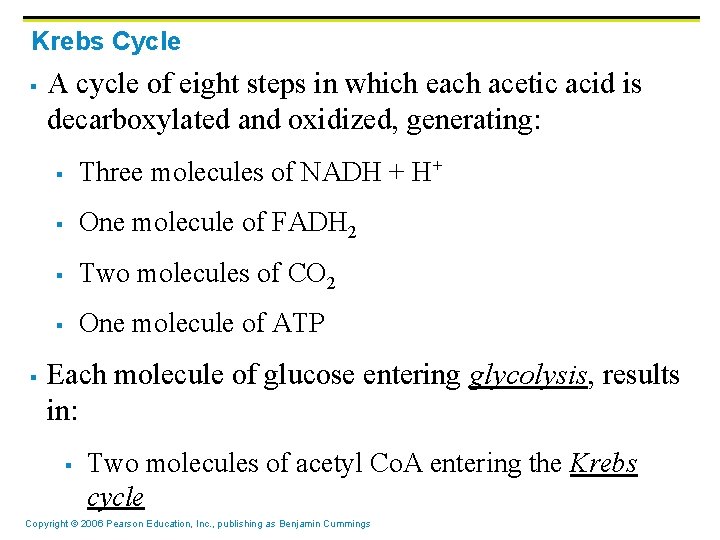
Krebs Cycle § § A cycle of eight steps in which each acetic acid is decarboxylated and oxidized, generating: § Three molecules of NADH + H+ § One molecule of FADH 2 § Two molecules of CO 2 § One molecule of ATP Each molecule of glucose entering glycolysis, results in: § Two molecules of acetyl Co. A entering the Krebs cycle Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

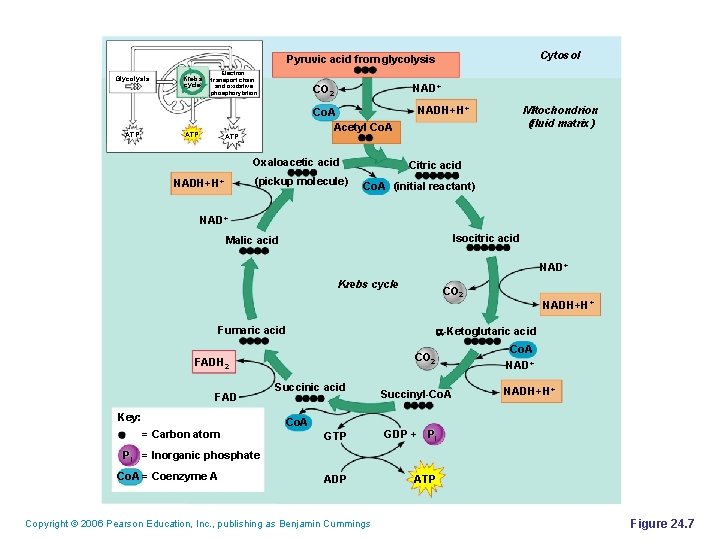
Cytosol Pyruvic acid from glycolysis Glycolysis ATP Krebs cycle Electron transport chain and oxidative phosphorylation ATP NAD+ CO 2 Co. A Acetyl Co. A ATP Oxaloacetic acid (pickup molecule) NADH+H+ Mitochondrion (fluid matrix) NADH+H+ Citric acid Co. A (initial reactant) NAD+ Isocitric acid Malic acid NAD+ Krebs cycle CO 2 Fumaric acid a-Ketoglutaric acid CO 2 FADH 2 FAD Key: = Carbon atom NADH+H+ Succinic acid Co. A Succinyl-Co. A GTP GDP + Pi ADP ATP Co. A NAD+ NADH+H+ Pi = Inorganic phosphate Co. A = Coenzyme A Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Figure 24. 7

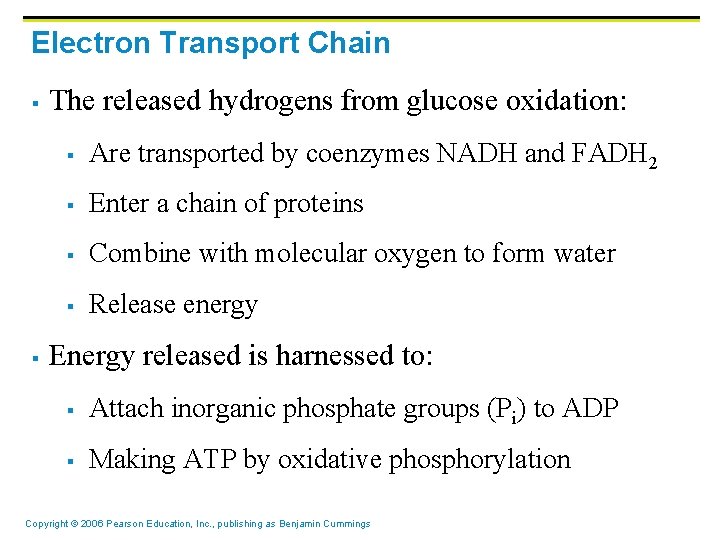
Electron Transport Chain § § The released hydrogens from glucose oxidation: § Are transported by coenzymes NADH and FADH 2 § Enter a chain of proteins § Combine with molecular oxygen to form water § Release energy Energy released is harnessed to: § Attach inorganic phosphate groups (Pi) to ADP § Making ATP by oxidative phosphorylation Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Mechanism of Oxidative Phosphorylation § hydrogens delivered to the chain are split into: § Protons (H+) and electrons § Protons are pumped across § § Inner mitochondrial membrane Electrons are shuttled from: § One acceptor to the next Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Mechanism of Oxidative Phosphorylation § Electrons are delivered to oxygen, forming oxygen ions § Oxygen ions attract H+ to form water § H+ pumped to the intermembrane space § Diffuses back to the matrix via ATP synthase § Releases energy § Energy is used to bond inorganic phosphate (Pi) to ADP producing ATP Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Summary of ATP Production Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Figure 24. 11

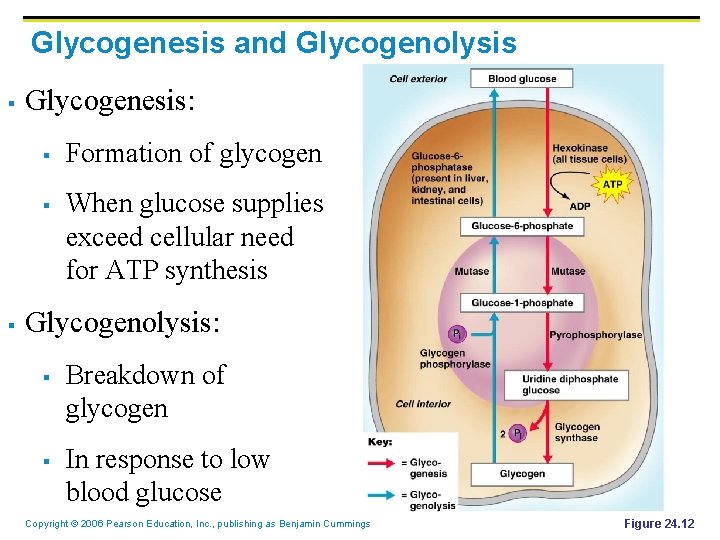
Glycogenesis and Glycogenolysis § Glycogenesis: § § § Formation of glycogen When glucose supplies exceed cellular need for ATP synthesis Glycogenolysis: § § Breakdown of glycogen In response to low blood glucose Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Figure 24. 12

Gluconeogenesis § Formation of sugar from non-carbohydrate molecules § Takes place mainly in the liver § Protects the body, especially the brain: § From damaging effects of hypoglycemia § By ensuring ATP synthesis can continue Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Lipid Metabolism § Pancraatic lipases digestion of lipids results in: § Free fatty acids (FFA) § Monoglycerides § Glycerol § FFA & monoglycerides are water insoluble § They quickly associate with: § § Bile salts (Polar & non-polar faces) § Lecithin (phospholipid) This association forms micelles Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

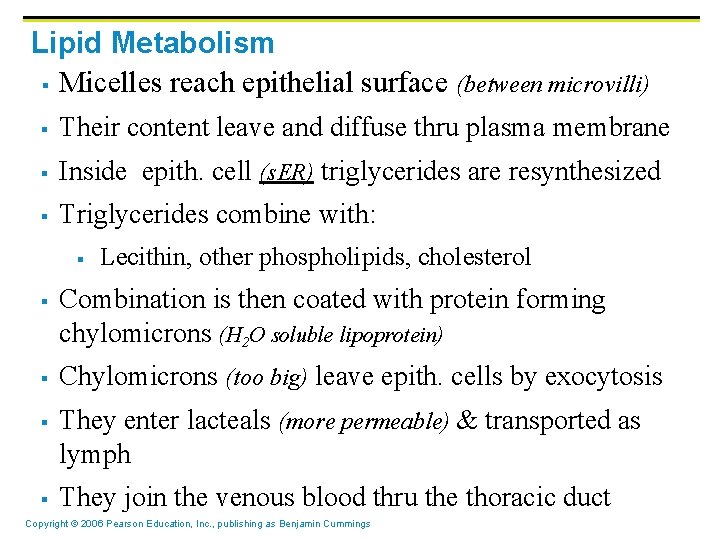
Lipid Metabolism § Micelles reach epithelial surface (between microvilli) § Their content leave and diffuse thru plasma membrane § Inside epith. cell (s. ER) triglycerides are resynthesized § Triglycerides combine with: § § § Lecithin, other phospholipids, cholesterol Combination is then coated with protein forming chylomicrons (H 2 O soluble lipoprotein) Chylomicrons (too big) leave epith. cells by exocytosis They enter lacteals (more permeable) & transported as lymph They join the venous blood thru the thoracic duct Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

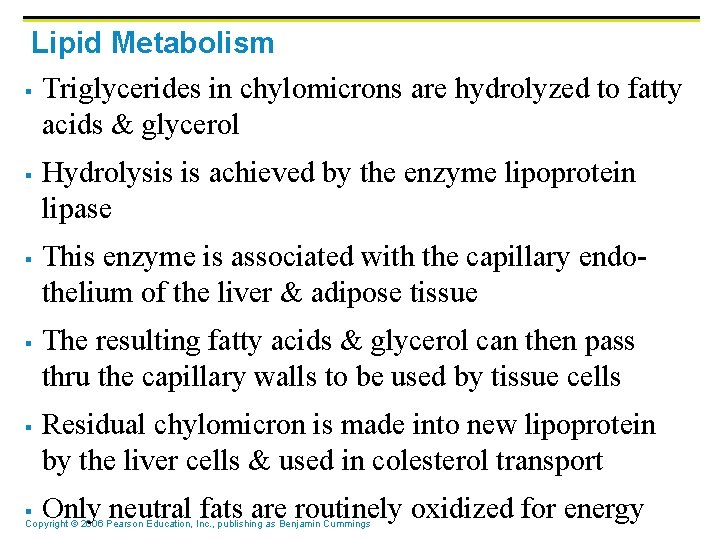
Lipid Metabolism § § § Triglycerides in chylomicrons are hydrolyzed to fatty acids & glycerol Hydrolysis is achieved by the enzyme lipoprotein lipase This enzyme is associated with the capillary endothelium of the liver & adipose tissue The resulting fatty acids & glycerol can then pass thru the capillary walls to be used by tissue cells Residual chylomicron is made into new lipoprotein by the liver cells & used in colesterol transport Only neutral fats are routinely oxidized for energy §Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

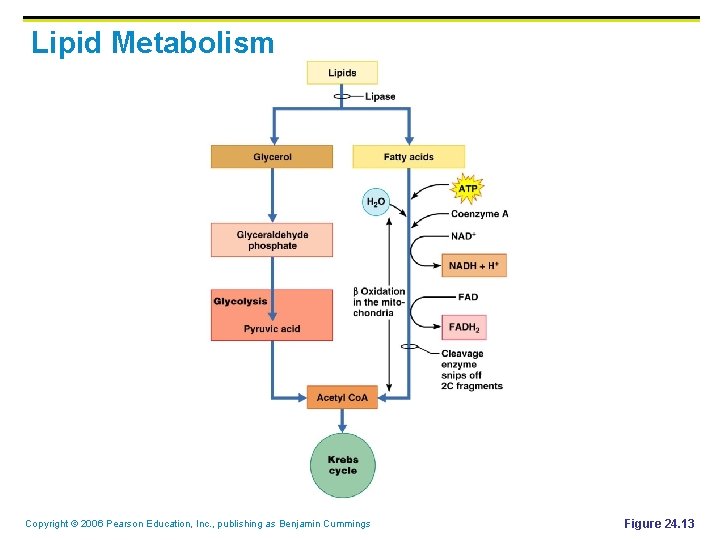
Lipid Metabolism § § Catabolism of fats involves two separate pathways § Glycerol pathway § Fatty acids pathway Glycerol is converted to: § Glyceraldehyde phosphate (GP) § GP is converted into acetyl Co. A § Acetyl Co. A enters the Krebs cycle § Energy (ATP) is produced Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Lipid Metabolism § Fatty acids undergo β-oxidation, which produces: § Two-carbon acetic acid fragments § These fragments enter the Krebs cycle § § § The resulting reduced coenzymes enter the electron transport chain Energy (ATP) is produced Short chain fatty acid from fat breakdown: § Don not follow the pathway described above § Simply diffuse into portal blood & be distributed Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Lipid Metabolism Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Figure 24. 13

Lipogenesis and Lipolysis § Lipogenesis: § § Conversion of excess dietary glycerol and fatty acids into triglycerides Glucose is easily converted into fat since acetyl Co. A is: § An intermediate in glucose catabolism § The starting molecule for fatty acid synthesis Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Lipogenesis and Lipolysis § Lipolysis: § The breakdown of stored fat § Is essentially lipogenesis in reverse Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Protein Metabolism § § Excess dietary protein results in: § Amino acids oxidation for energy § Convertion of amino acids into fat for storage Amino acids must be: § § § Deaminated prior to oxidation for energy Deaminated amino acids are converted into: § Pyruvic acid, or § One of the intermediate keto acids These acids are intermediates in the Krebs cycle Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Liver Functions: § A brief summary of liver functions: § Packages fatty acids to be stored and transported § Synthesizes plasma proteins § Forms nonessential amino acids § Converts deamination ammonia into urea § Stores glucose as glycogen § Regulates blood glucose homeostasis § Stores vitamins, § Detoxifies substances Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

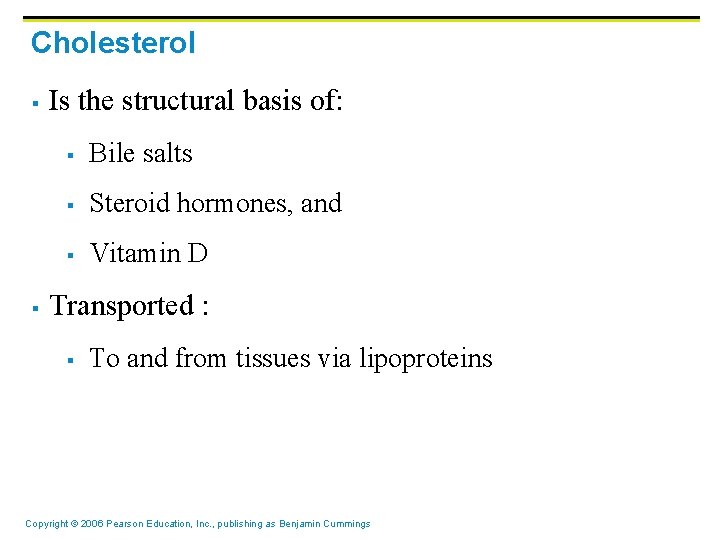
Cholesterol § § Is the structural basis of: § Bile salts § Steroid hormones, and § Vitamin D Transported : § To and from tissues via lipoproteins Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

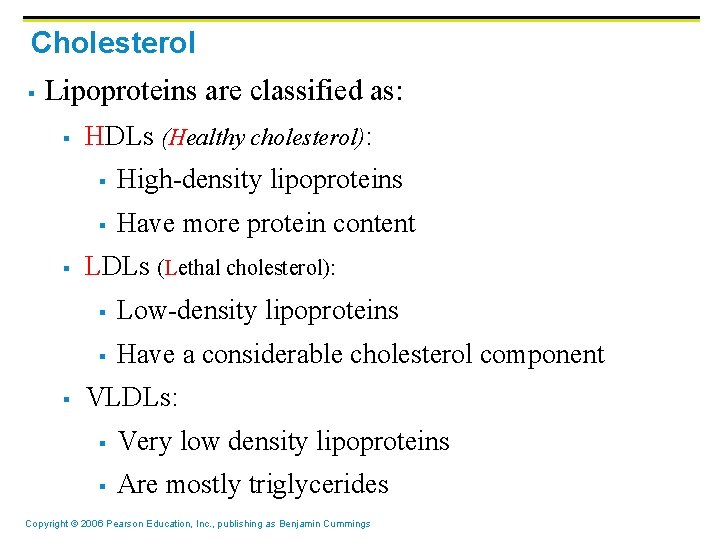
Cholesterol § Lipoproteins are classified as: § § § HDLs (Healthy cholesterol): § High-density lipoproteins § Have more protein content LDLs (Lethal cholesterol): § Low-density lipoproteins § Have a considerable cholesterol component VLDLs: § Very low density lipoproteins § Are mostly triglycerides Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

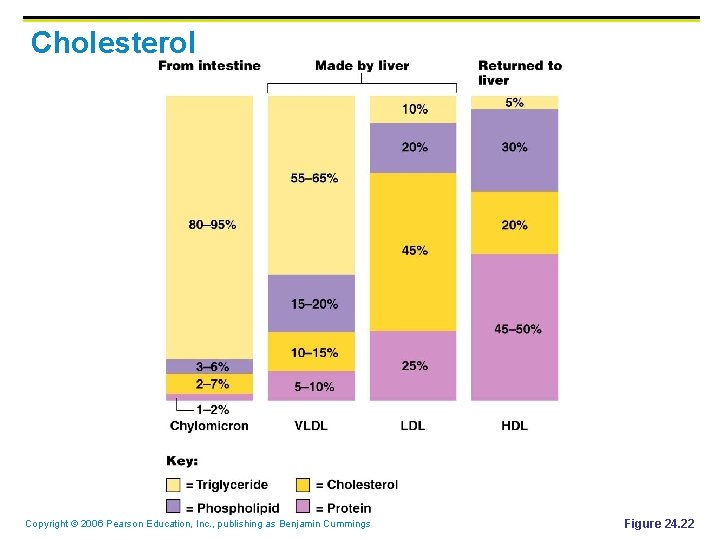
Cholesterol Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings Figure 24. 22

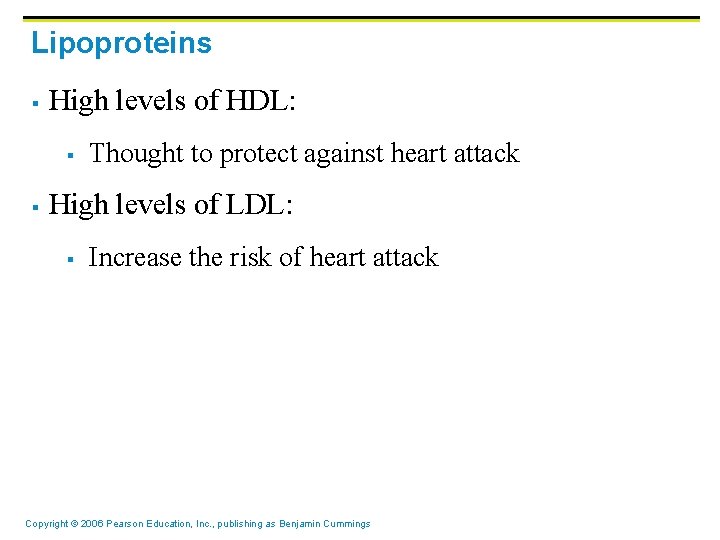
Lipoproteins § High levels of HDL: § § Thought to protect against heart attack High levels of LDL: § Increase the risk of heart attack Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

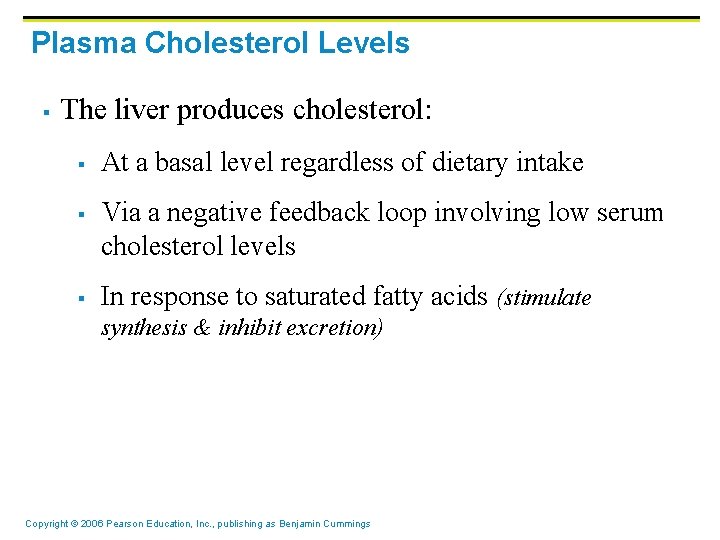
Plasma Cholesterol Levels § The liver produces cholesterol: § § § At a basal level regardless of dietary intake Via a negative feedback loop involving low serum cholesterol levels In response to saturated fatty acids (stimulate synthesis & inhibit excretion) Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

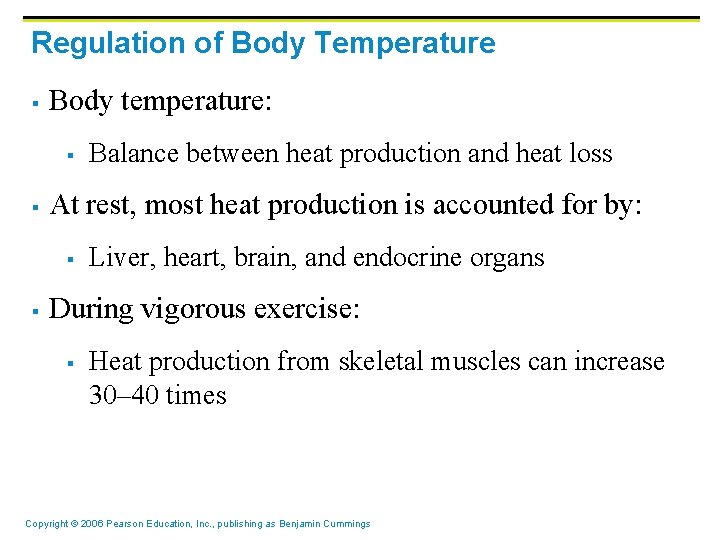
Regulation of Body Temperature § Body temperature: § § At rest, most heat production is accounted for by: § § Balance between heat production and heat loss Liver, heart, brain, and endocrine organs During vigorous exercise: § Heat production from skeletal muscles can increase 30– 40 times Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

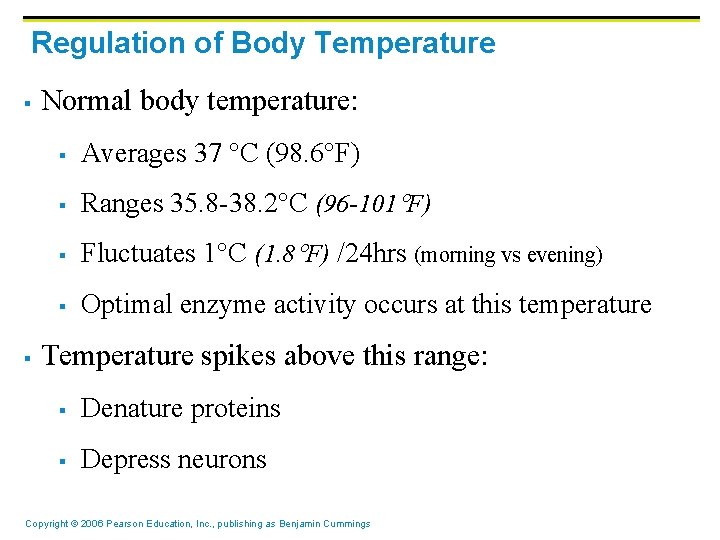
Regulation of Body Temperature § § Normal body temperature: § Averages 37 C (98. 6 F) § Ranges 35. 8 -38. 2 C (96 -101 F) § Fluctuates 1 C (1. 8 F) /24 hrs (morning vs evening) § Optimal enzyme activity occurs at this temperature Temperature spikes above this range: § Denature proteins § Depress neurons Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Core and Shell Temperature § § Core organs (have the highest temperature) are found: § Within the skull § Thoracic cavity § Abdominal cavity The shell (has the lowest temperature) : § § Major agent of heat transfer between the core and shell: § § Blood Core temperature: § § Essentially the skin Remains relatively constant Shell temperature: § Fluctuates substantially (20– 40 C) Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

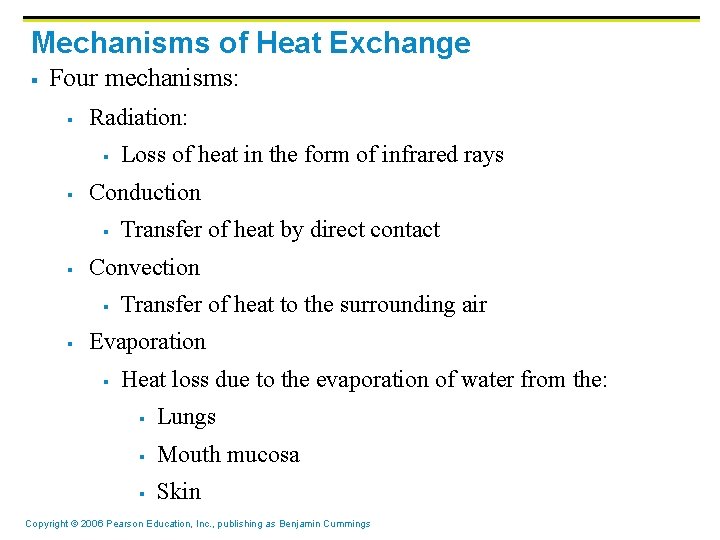
Mechanisms of Heat Exchange § Four mechanisms: § Radiation: § § Conduction § § Transfer of heat by direct contact Convection § § Loss of heat in the form of infrared rays Transfer of heat to the surrounding air Evaporation § Heat loss due to the evaporation of water from the: § Lungs § Mouth mucosa § Skin Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Heat-Promoting Mechanisms § § Stimuli: § Low external temperature § Low temperature of circulating blood Heat-promoting centers (hypothalamus) cause: § Vasoconstriction of cutaneous blood vessels § Increased metabolic rate § Shivering § Enhanced thyroxine release Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings

Heat-Loss Mechanisms § Stimulus: § § § Core temperature rises: Heat-loss center is activated to cause: § Vasodilation of cutaneous blood vessels § Enhanced sweating Voluntary measures to reduce body heat: § Reduce activity § Seek a cooler environment § Wear light-colored & loose-fitting clothing Copyright © 2006 Pearson Education, Inc. , publishing as Benjamin Cummings