Glycolysis and Gluconeogenesis The Journey to Pyruvate and
























- Slides: 74

Glycolysis and Gluconeogenesis The Journey to Pyruvate and Back Again Dr Anwar Matar Almaeene School of Pharmacy, Keele University 1

Lecture 2 Outcomes • Name the compounds and intermediates involved in glycolysis and gluconeogenesis and draw their structures • List the enzymatic reactions in these processes, name the enzymes involved and draw the complete pathways • Know the control points in these processes and name the activators and inhibitors that regulate them • Material covered in Berg et al Chapter 16, 20 & 27 2

Major Metabolic Pathways 3

How It Happens - Thermodynamics • Glycolytic and glucogenetic pathways obey the same basic laws of thermodynamics • Gibb’s Free Energy �G = �H - T�S • Use �G and �Gº′ to analyze metabolic processes • A reaction will take place if �G is –ve • If �G is +ve there is no spontaneous reaction • No net change if �G = 0, i. e. equilibrium • Reactions can be coupled to drive the process 4

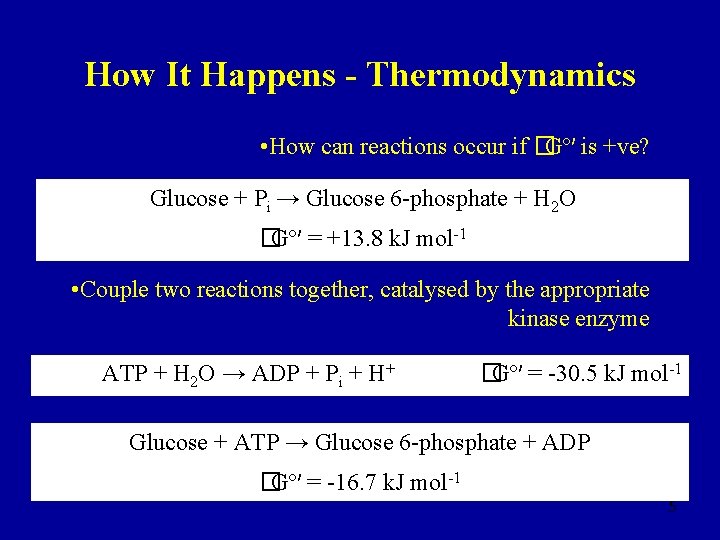
How It Happens - Thermodynamics • How can reactions occur if �Gº′ is +ve? Glucose + Pi → Glucose 6 -phosphate + H 2 O �Gº′ = +13. 8 k. J mol-1 • Couple two reactions together, catalysed by the appropriate kinase enzyme ATP + H 2 O → ADP + Pi + H+ �Gº′ = -30. 5 k. J mol-1 Glucose + ATP → Glucose 6 -phosphate + ADP �Gº′ = -16. 7 k. J mol-1 5

Carbohydrate Metabolism Facts • Glycosidic bond conformations are important • Starch: �-1, 4 • Glycogen: �-1, 4 and �-1, 6 • Cellulose: �-1, 4 • Enzymes are usually specific for a particular carbohydrate and glycosidic bond conformation • e. g. �-1, 4 glucosidase vs �-1, 4 glucosidase • Many carbohydrates are metabolised as the phosphates, e. g. glucose 6 -phosphate 6

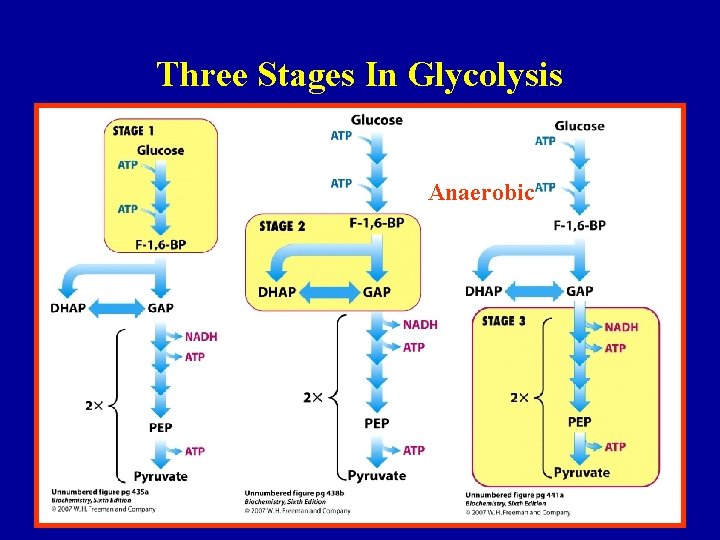
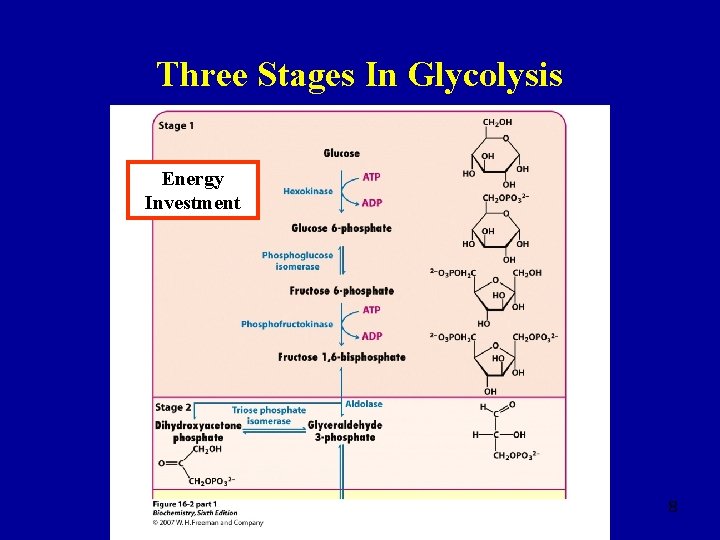
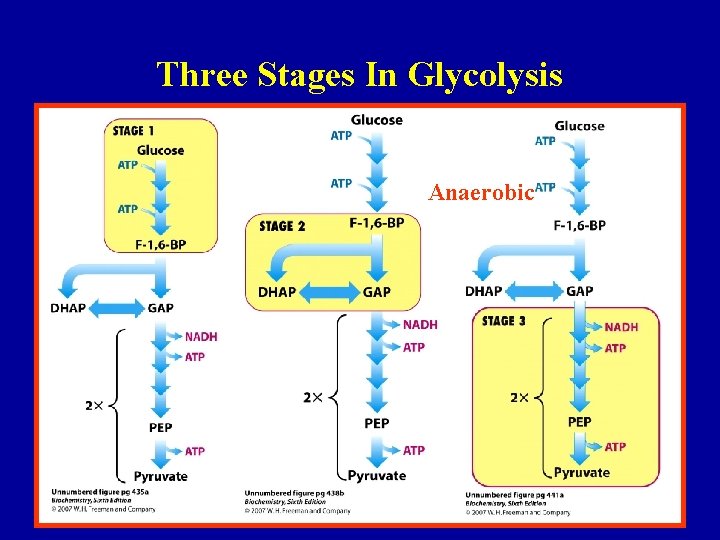
Three Stages In Glycolysis Anaerobic 7

Three Stages In Glycolysis Energy Investment 8

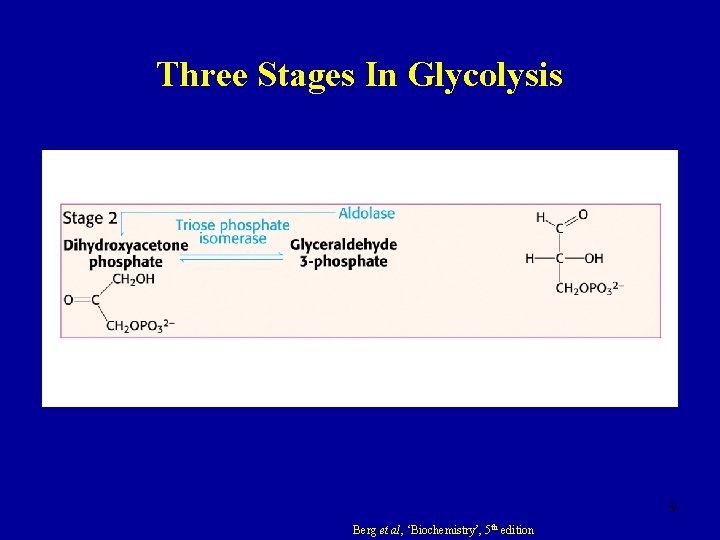
Three Stages In Glycolysis 9 Berg et al, ‘Biochemistry’, 5 th edition

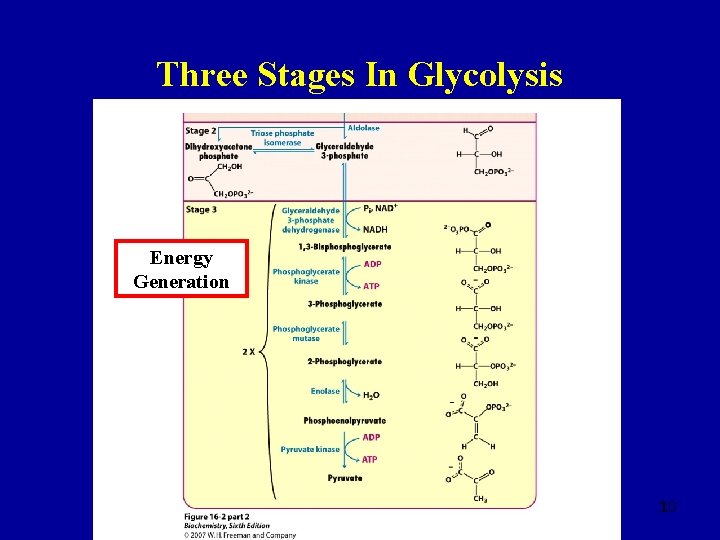
Three Stages In Glycolysis Energy Generation 10

Glycolysis Facts • Catabolic convergence: glucose, galactose, fructose and others can feed into the process • No loss of carbon atoms occurs • 4 hydrogen atoms are lost (NAD+ involved) • No molecular oxygen is involved • Will compare �G throughout, remember that the value of �Gº′ can be +ve while �G is -ve 11

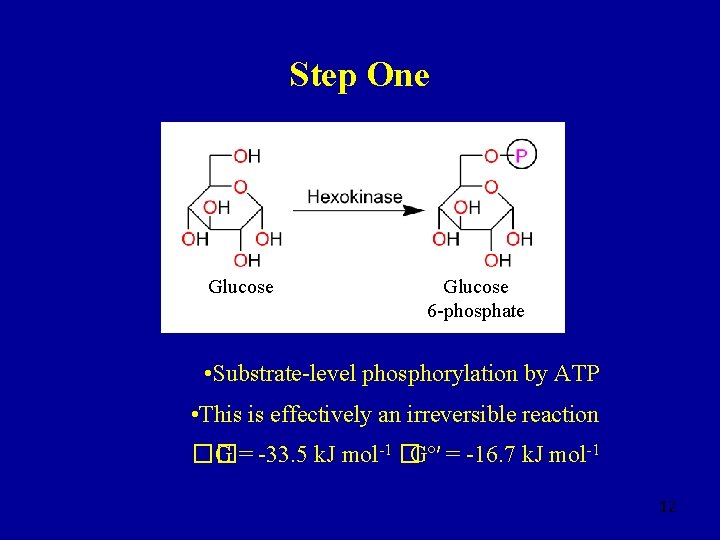
Step One Glucose 6 -phosphate • Substrate-level phosphorylation by ATP • This is effectively an irreversible reaction �� G = -33. 5 k. J mol-1 �Gº′ = -16. 7 k. J mol-1 12

Why Do This? • Glucose: polar, neutral • Glucose 6 -phosphate: polar, -ve charge • Intracellular Glc readily converted to Glc-6 P • Glucose 6 -phosphate is trapped inside cells as it cannot penetrate plasma membranes • [ATP]/[ADP] >1 + [Glc]/[Glc-6 P] >1 drives reaction 13

Hexokinase • Found in all cells in the body • Kinase: an enzyme that adds a phosphate group to the substrate using ATP as the donor • Hexo: denotes C 6 carbohydrates are the substrate • Glc, Gal, Fru and other hexoses are substrates • Glucokinase (liver) reacts with Glc only • Both of the enzymes phosphorylate carbohydrates at the 6 position 14

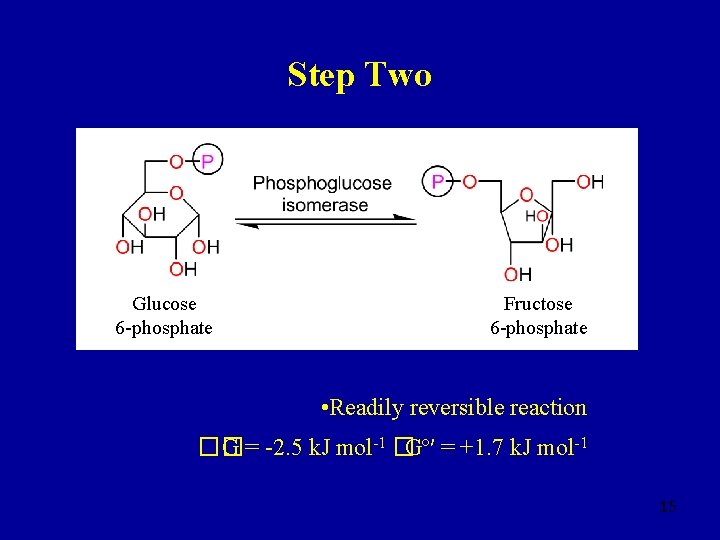
Step Two Glucose 6 -phosphate Fructose 6 -phosphate • Readily reversible reaction �� G = -2. 5 k. J mol-1 �Gº′ = +1. 7 k. J mol-1 15

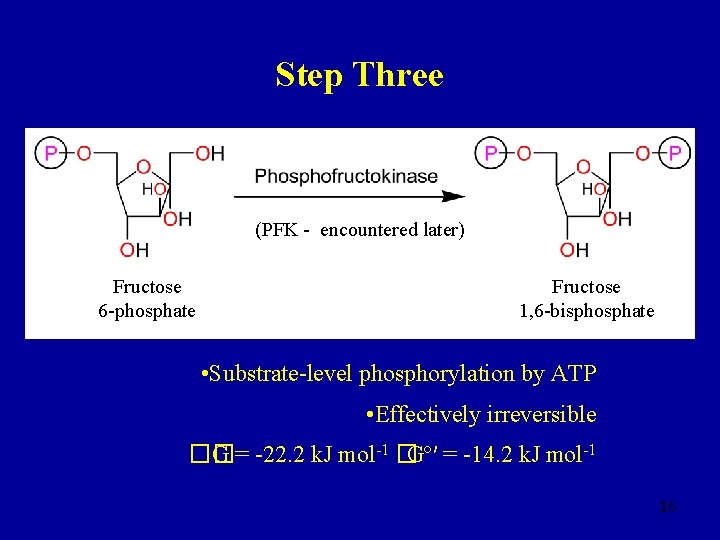
Step Three (PFK - encountered later) Fructose 6 -phosphate Fructose 1, 6 -bisphosphate • Substrate-level phosphorylation by ATP • Effectively irreversible �� G = -22. 2 k. J mol-1 �Gº′ = -14. 2 k. J mol-1 16

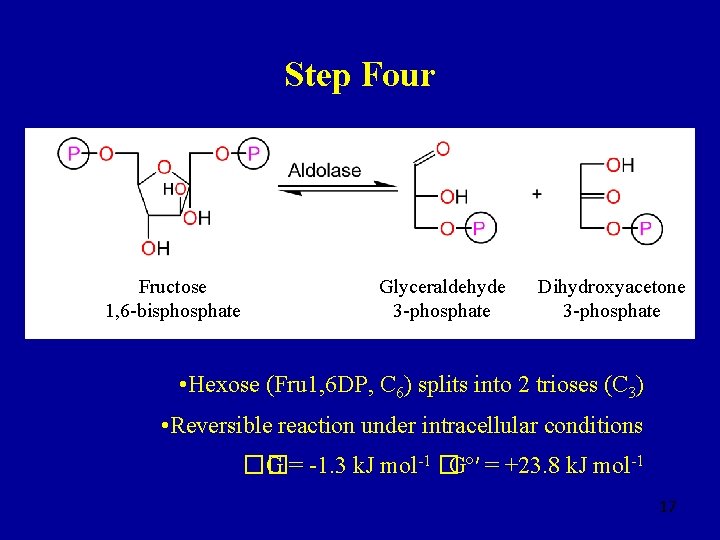
Step Four Fructose 1, 6 -bisphosphate Glyceraldehyde 3 -phosphate Dihydroxyacetone 3 -phosphate • Hexose (Fru 1, 6 DP, C 6) splits into 2 trioses (C 3) • Reversible reaction under intracellular conditions �� G = -1. 3 k. J mol-1 �Gº′ = +23. 8 k. J mol-1 17

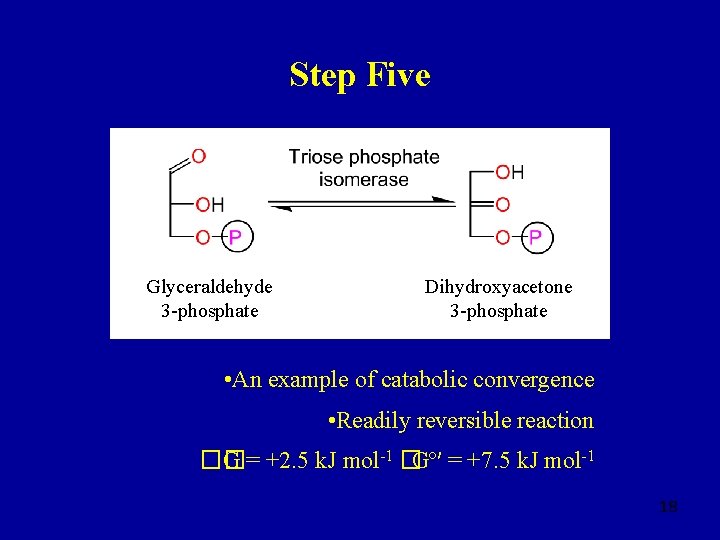
Step Five Glyceraldehyde 3 -phosphate Dihydroxyacetone 3 -phosphate • An example of catabolic convergence • Readily reversible reaction �� G = +2. 5 k. J mol-1 �Gº′ = +7. 5 k. J mol-1 18

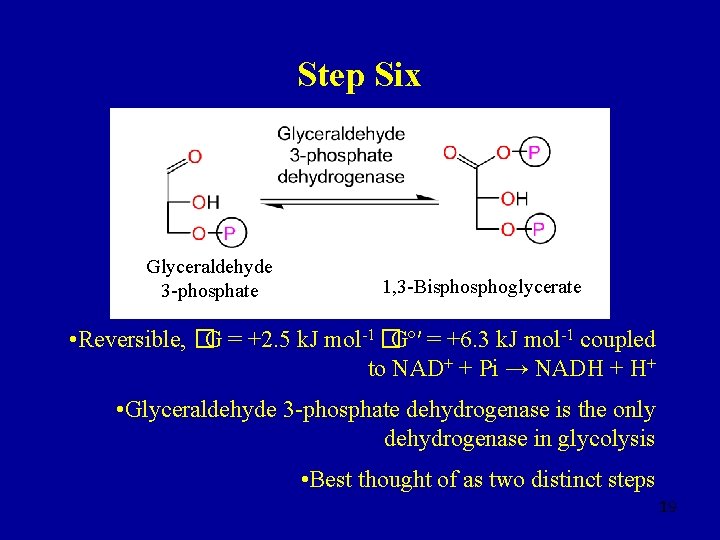
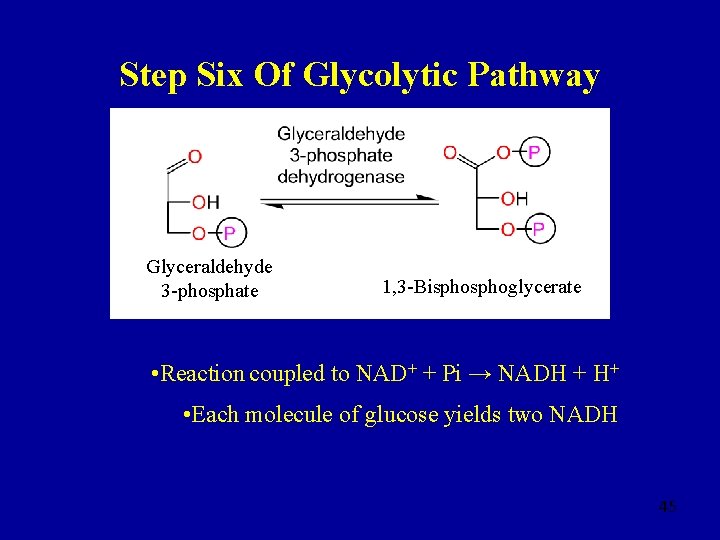
Step Six Glyceraldehyde 3 -phosphate 1, 3 -Bisphoglycerate • Reversible, �G = +2. 5 k. J mol-1 �Gº′ = +6. 3 k. J mol-1 coupled to NAD+ + Pi → NADH + H+ • Glyceraldehyde 3 -phosphate dehydrogenase is the only dehydrogenase in glycolysis • Best thought of as two distinct steps 19

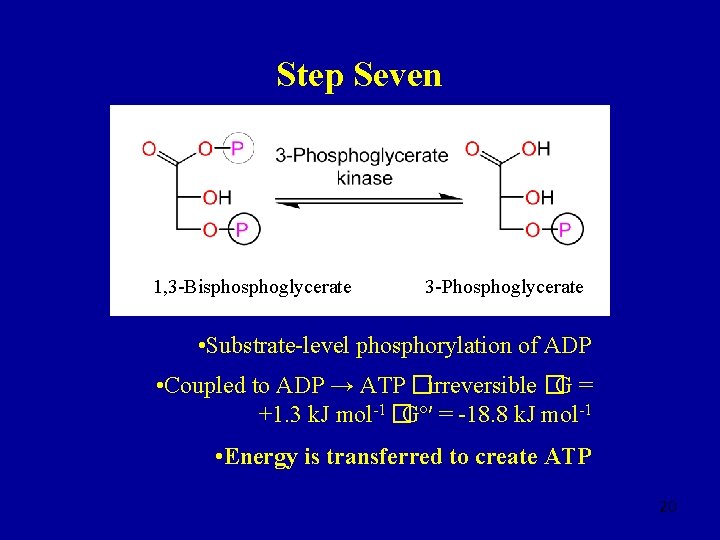
Step Seven 1, 3 -Bisphoglycerate 3 -Phosphoglycerate • Substrate-level phosphorylation of ADP • Coupled to ADP → ATP �irreversible �G = +1. 3 k. J mol-1 �Gº′ = -18. 8 k. J mol-1 • Energy is transferred to create ATP 20

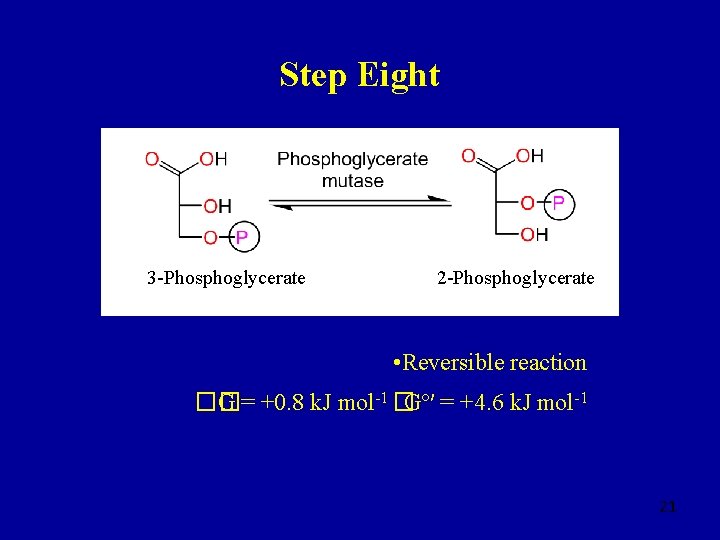
Step Eight 3 -Phosphoglycerate 2 -Phosphoglycerate • Reversible reaction �� G = +0. 8 k. J mol-1 �Gº′ = +4. 6 k. J mol-1 21

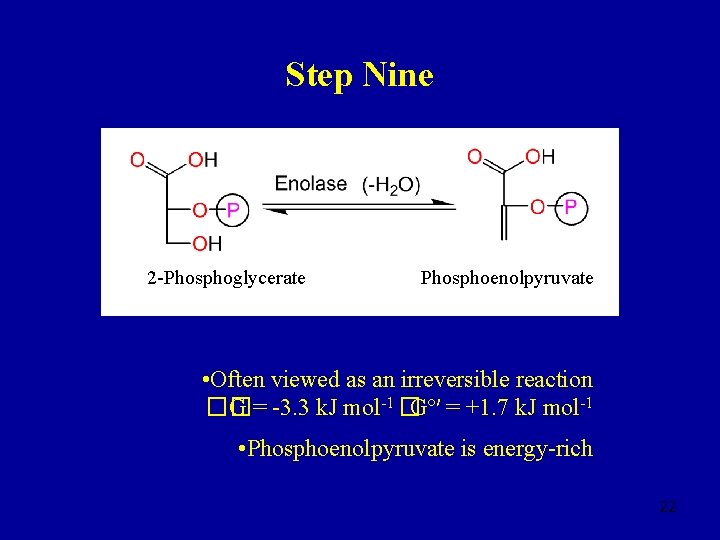
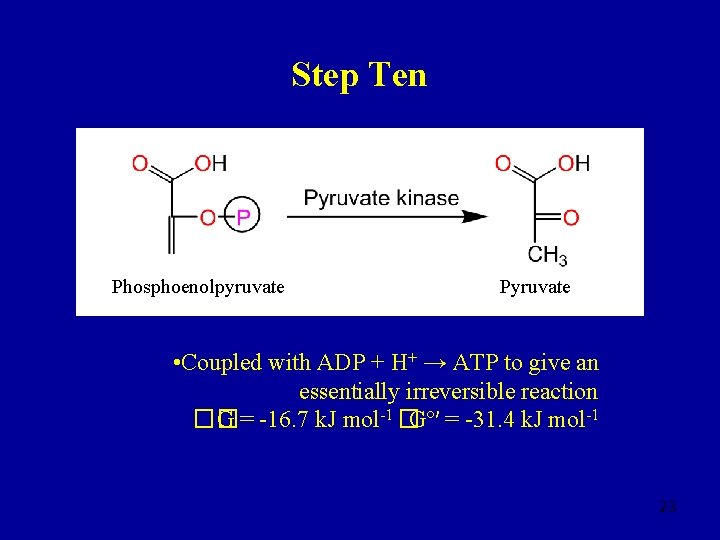
Step Nine 2 -Phosphoglycerate Phosphoenolpyruvate • Often viewed as an irreversible reaction �� G = -3. 3 k. J mol-1 �Gº′ = +1. 7 k. J mol-1 • Phosphoenolpyruvate is energy-rich 22

Step Ten Phosphoenolpyruvate Pyruvate • Coupled with ADP + H+ → ATP to give an essentially irreversible reaction �� G = -16. 7 k. J mol-1 �Gº′ = -31. 4 k. J mol-1 23

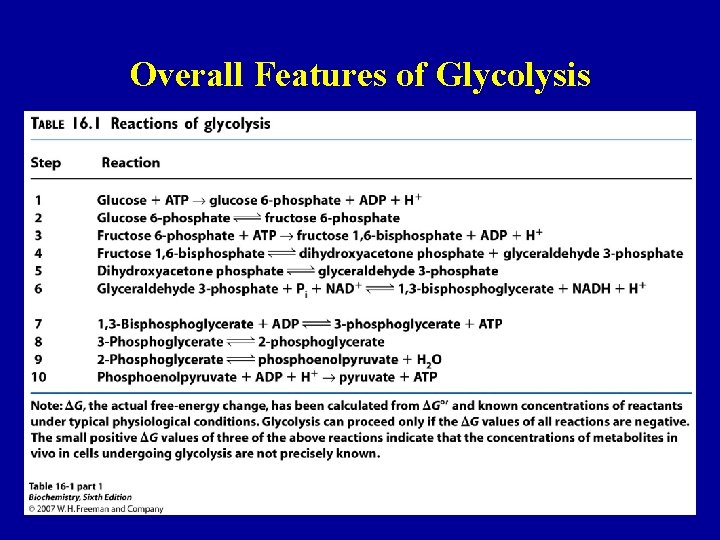
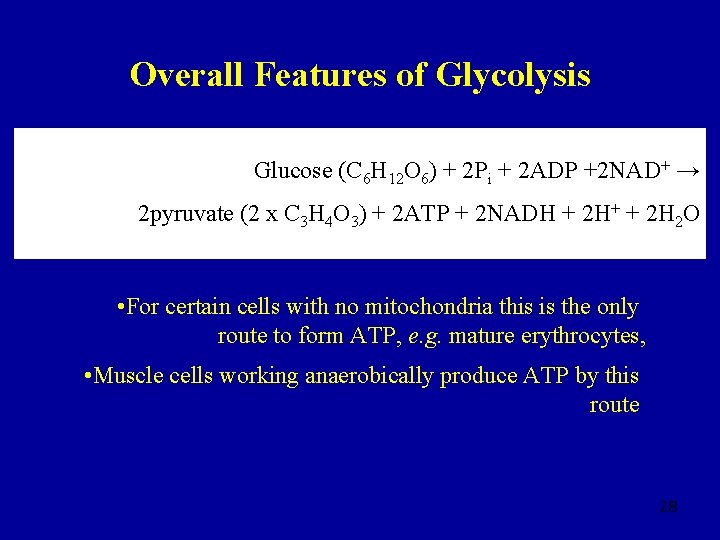
Overall Features of Glycolysis 24

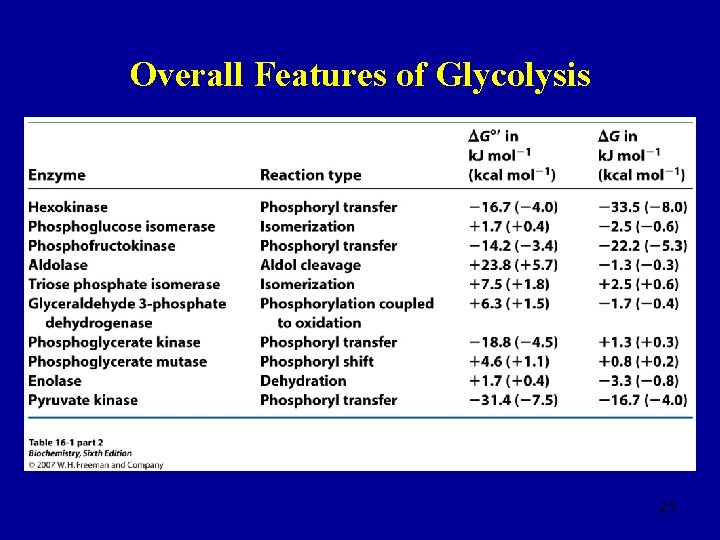
Overall Features of Glycolysis 25

Overall Features of Glycolysis • The total energy released ~200 k. J mol-1 • This is only a small fraction of the total released by the complete oxidation of a molecule of glucose (~2900 k. J mol -1) • Three reactions (1, 3 and 10) show a big release of energy, make glycolysis irreversible • Pyruvate is converted to acetyl Co. A within the mitochondrion, this is also irreversible 26

Overall Features of Glycolysis • All reactions take place within the cytosol • Four reactions involve kinases • Hexokinase (ATP yield -1) • Phosphofructokinase (ATP yield -1) • Phosphoglycerate kinase (ATP yield +2) • Pyruvate kinase (ATP yield +2) • Overall yield of ATP per mole glucose = +2 27

Overall Features of Glycolysis Glucose (C 6 H 12 O 6) + 2 Pi + 2 ADP +2 NAD+ → 2 pyruvate (2 x C 3 H 4 O 3) + 2 ATP + 2 NADH + 2 H+ + 2 H 2 O • For certain cells with no mitochondria this is the only route to form ATP, e. g. mature erythrocytes, • Muscle cells working anaerobically produce ATP by this route 28

Overall Features of Glycolysis • Overall yield of NADH per mole glucose = +2 (corresponds to 4 H atoms) • Under aerobic conditions this NADH is reoxidised by O 2 in the mitochondria to give NAD+ and lactate • By forming lactate from pyruvate an anaerobic cell can avoid running short of NAD+, keeping glycolysis running in the absence of oxygen 29

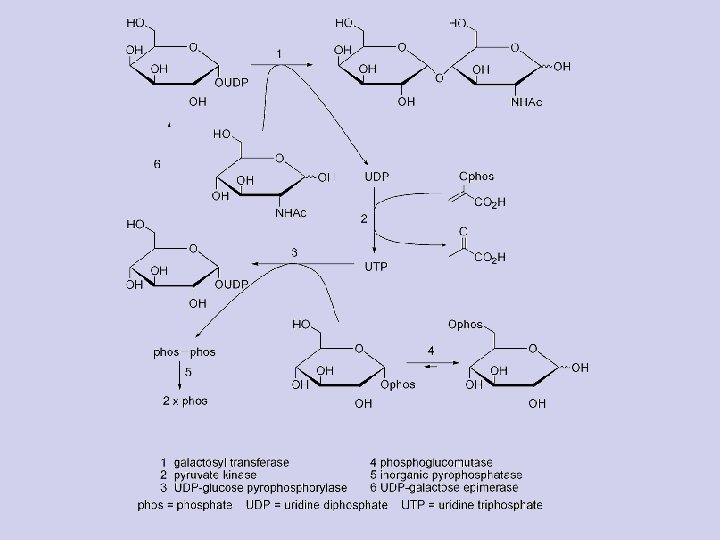
Galactose: (from Greek galaktos "milk"), sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is considered a nutritive sweetener because it has food energy - Galactose is a monosaccharide. When combined with glucose, through a dehydration reaction, the result is the disaccharide lactose. The hydrolysis of lactose to glucose and galactose is catalyzed by the enzyme lactase and β-galactosidase. - Galactose and glucose are the two monosaccharide sugar components that make up the disaccharide sugar, lactose. Lactose is found primarily in milk and milk products. Galactose metabolism, which converts galactose into glucose, is carried out by the three principal enzymes in a mechanism known as the Leloir Pathway. The enzymes are listed in the order of the metabolic pathway: galactokinase (GALK), galactose-1 -phosphate uridyltransferase (GALT), and UDP-galactose-4’-epimerase (GALE).


Galactose and Fructose 32

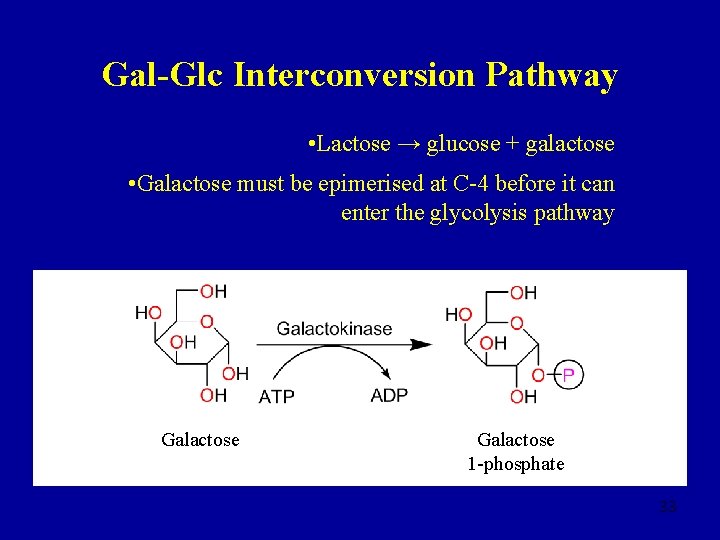
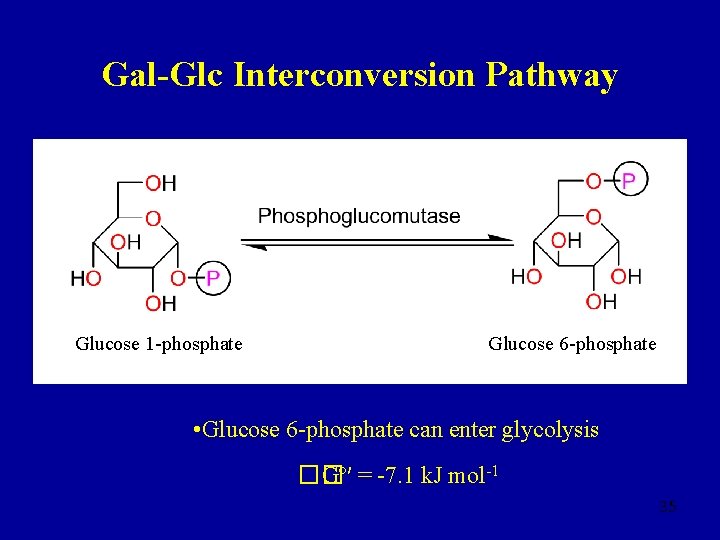
Gal-Glc Interconversion Pathway • Lactose → glucose + galactose • Galactose must be epimerised at C-4 before it can enter the glycolysis pathway Galactose 1 -phosphate 33

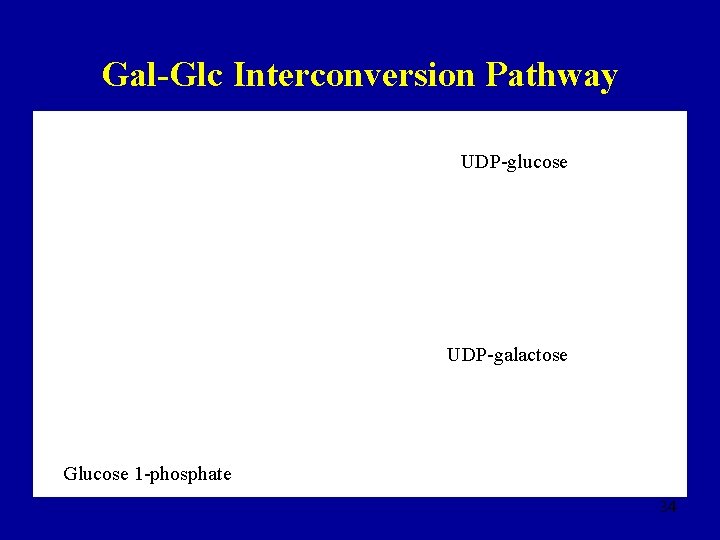
Gal-Glc Interconversion Pathway UDP-glucose UDP-galactose Glucose 1 -phosphate 34

Gal-Glc Interconversion Pathway Glucose 1 -phosphate Glucose 6 -phosphate • Glucose 6 -phosphate can enter glycolysis �� Gº′ = -7. 1 k. J mol-1 35

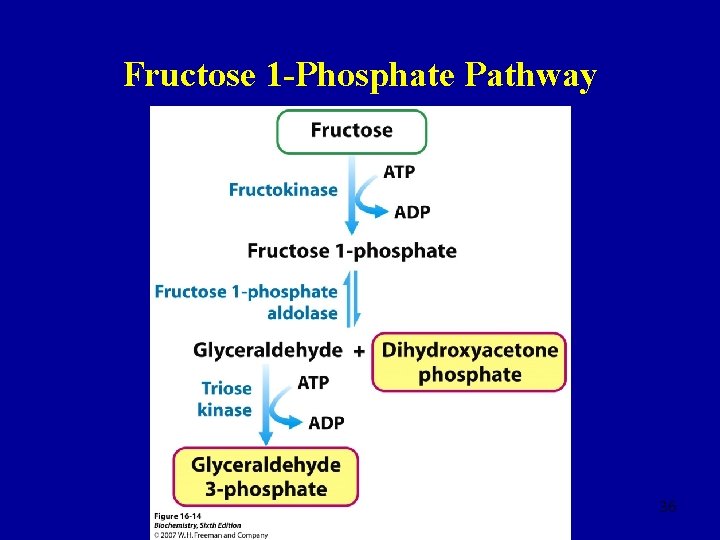
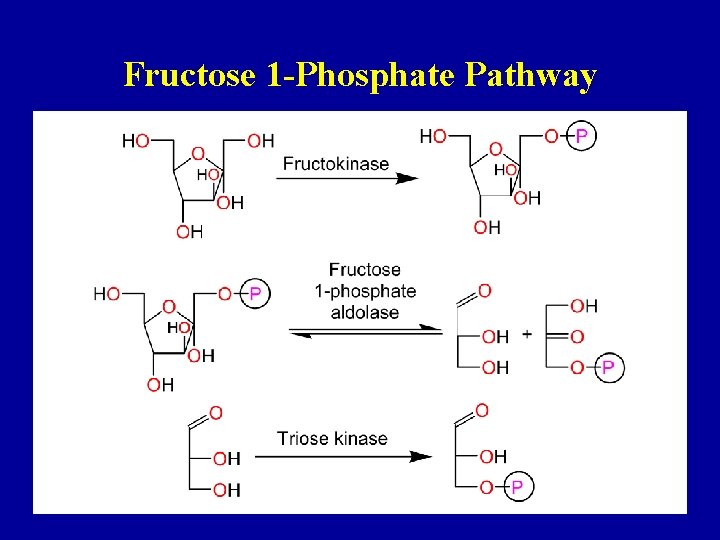
Fructose 1 -Phosphate Pathway 36

Fructose 1 -Phosphate Pathway 37

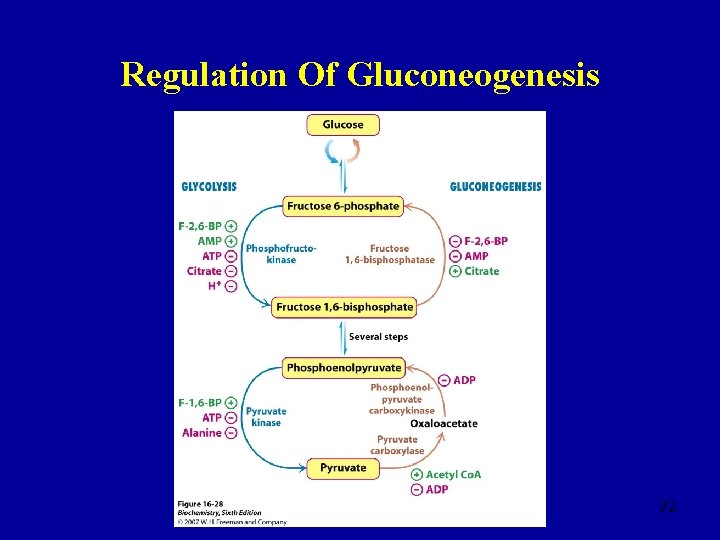
Regulation Of Glycolysis – Phosphofructokinase (PFK) • Allosterically inhibited by ATP, reversed by AMP • Allows glycolysis to respond to ATP deficiency • Inhibited by citrate, product of the TCA cycle • No further breakdown of glucose is required • Inhibited by low p. H • Prevents excessive formation of lactate under anaerobic conditions • Regulation by fructose 2, 6 -bisphosphate 38

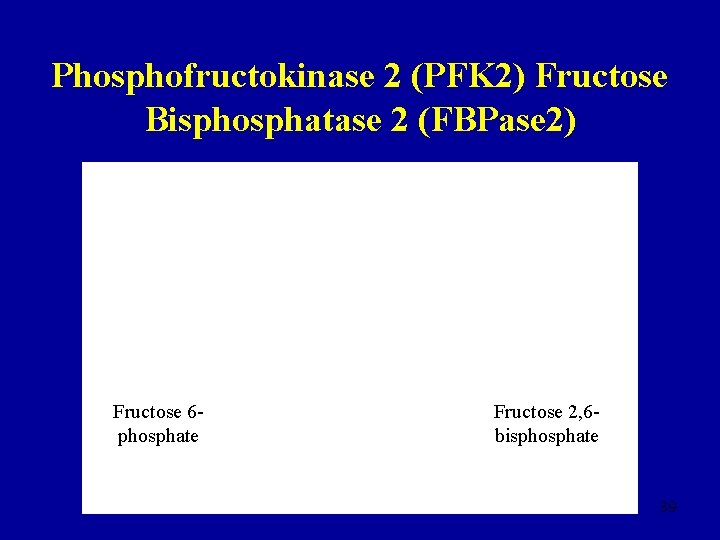
Phosphofructokinase 2 (PFK 2) Fructose Bisphosphatase 2 (FBPase 2) Fructose 6 phosphate Fructose 2, 6 bisphosphate 39

Fructose 2, 6 -bisphosphate • Fructose 6 -phosphate stimulates the synthesis of fructose 2, 6 -bisphosphate by PFK 2 and inhibits hydrolysis by FBPase 2 • Fructose 2, 6 -bisphosphate activates PFK • High levels of fructose 6 -phosphate increase the rate of glycolysis • [Glucose]↓, [fructose 2, 6 -bisphosphate]↓ and the rate of glycolysis decreases • [Glucose]↑, [fructose 2, 6 -bisphosphate]↑ and the rate of glycolysis increases 40

Regulation Of Glycolysis - Hexokinase • Inhibited by glucose 6 -phosphate • When PFK is inhibited [fructose 6 -phosphate]↑ and [glucose 6 phosphate]↑ • This is not the primary control point in the glycolytic pathway, despite being an irreversible step in the process • Glucose 6 -phosphate takes part in glycogen synthesis and the pentose phosphate pathway • The first irreversible step unique to glycolysis is catalysed by PFK �this is the primary control 41

Regulation Of Glycolysis – Pyruvate Kinase • Activated by fructose 1, 6 -bisphosphate • Inhibited when [glucose]↓ • Inhibited by high levels of ATP 42

Three Stages In Glycolysis Anaerobic 43

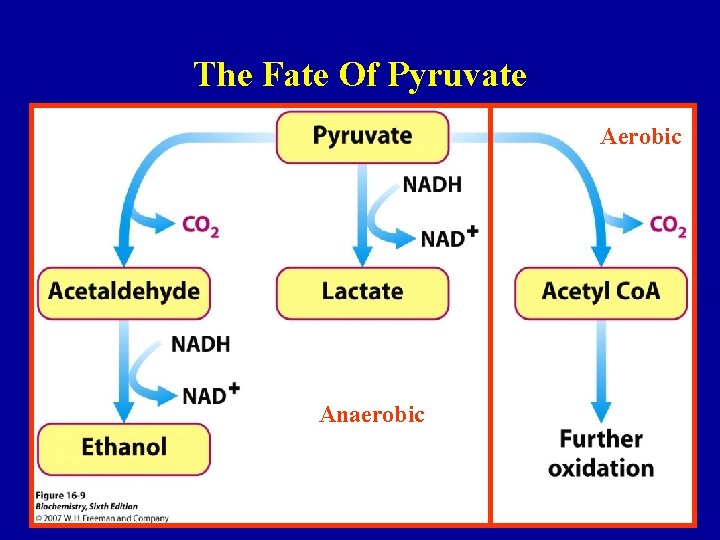
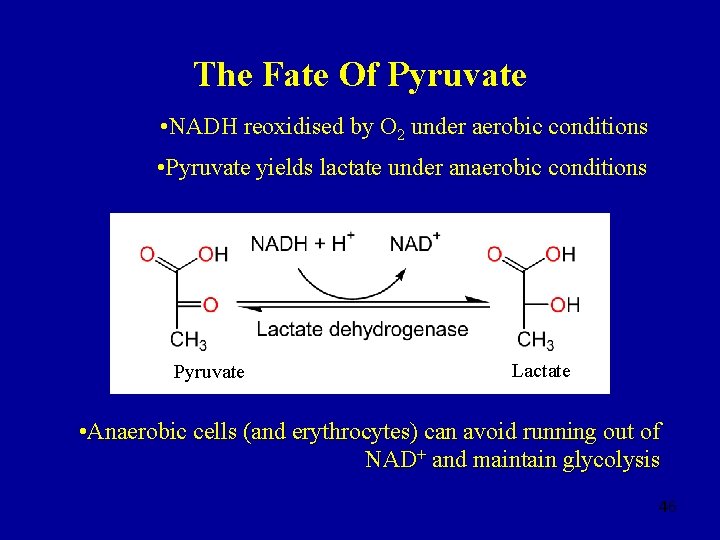
The Fate Of Pyruvate Aerobic Anaerobic 44

Step Six Of Glycolytic Pathway Glyceraldehyde 3 -phosphate 1, 3 -Bisphoglycerate • Reaction coupled to NAD+ + Pi → NADH + H+ • Each molecule of glucose yields two NADH 45

The Fate Of Pyruvate • NADH reoxidised by O 2 under aerobic conditions • Pyruvate yields lactate under anaerobic conditions Pyruvate Lactate • Anaerobic cells (and erythrocytes) can avoid running out of NAD+ and maintain glycolysis 46

The Fate Of Pyruvate • In highly active muscle tissue the amount of pyruvate produced can exceed the ability of the TCA cycle to oxidise it by aerobic oxidation • Energy can be produced anaerobically from glycogen stores but only for a short period • Rapid anaerobic metabolism of glucose leads to increased levels of lactate (lowers blood p. H) • Anaerobic metabolism yields 2 x lactate + 2 ATP • Aerobic metabolism yields 6 CO 2 + 6 H 2 O + 38 ATP • Muscles cannot metabolise lactate further 47

The Fate Of Lactate • What happens to lactate released into the blood? • How do the brain and erythrocytes obtain an adequate supply of glucose during periods of prolonged exercise or starvation? • Glucose is required for normal brain and erythrocyte function • Erythrocytes have no mitochondria, so cannot use molecular O 2 as oxidant �anaerobic • Erythrocytes produce lactate even under non-stressful circumstances 48

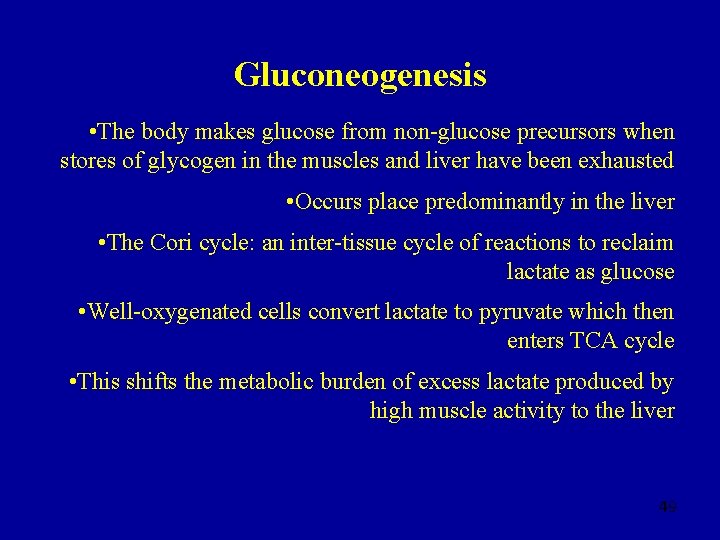
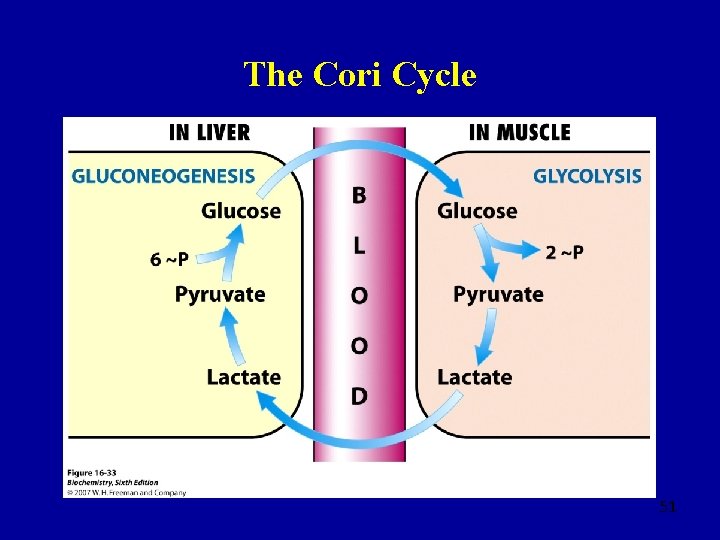
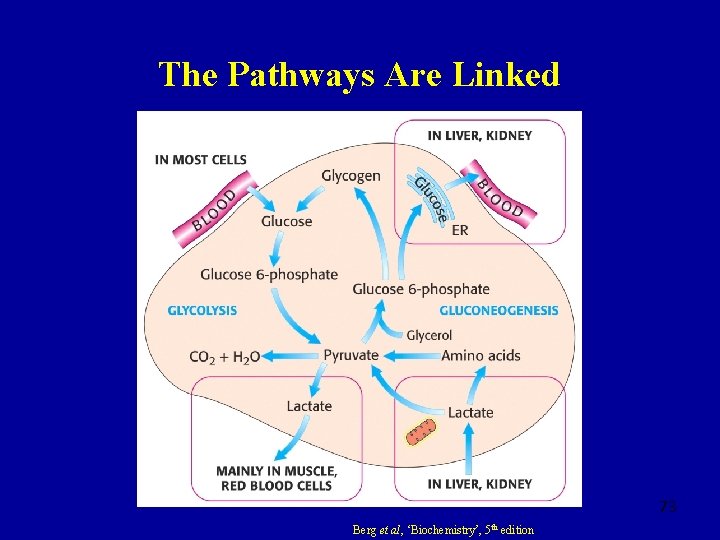
Gluconeogenesis • The body makes glucose from non-glucose precursors when stores of glycogen in the muscles and liver have been exhausted • Occurs place predominantly in the liver • The Cori cycle: an inter-tissue cycle of reactions to reclaim lactate as glucose • Well-oxygenated cells convert lactate to pyruvate which then enters TCA cycle • This shifts the metabolic burden of excess lactate produced by high muscle activity to the liver 49

gluconeogenesis provides a substantial fraction of the glucose produced in fasting human even after a few hours fast. Gluconeogenesis occurs in liver and to a smaller extent in kidney. The noncarbohydrate precursors that can be converted to glucose include the glycolysis products lactate and pyruvate, citric acid cycle intermediates, and the carbon skeletons of most amino acids. However, all these substances must be converted to oxaloacetate, the starting material for gluconeogenesis.

The Cori Cycle 51

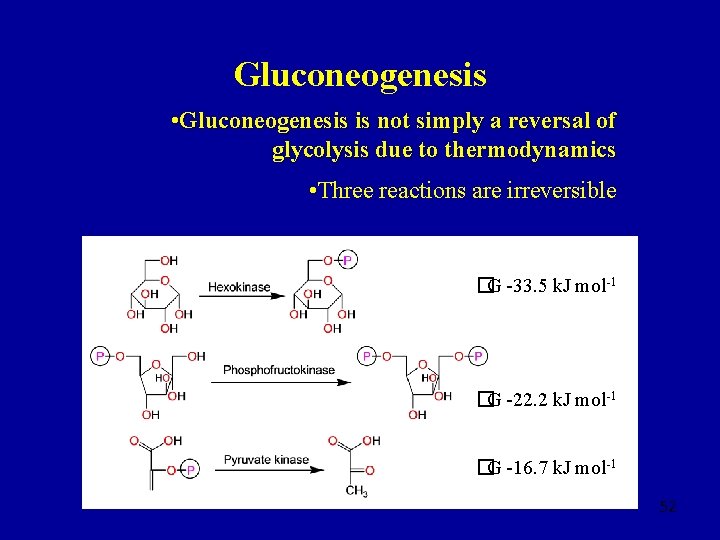
Gluconeogenesis • Gluconeogenesis is not simply a reversal of glycolysis due to thermodynamics • Three reactions are irreversible �G -33. 5 k. J mol-1 �G -22. 2 k. J mol-1 �G -16. 7 k. J mol-1 52

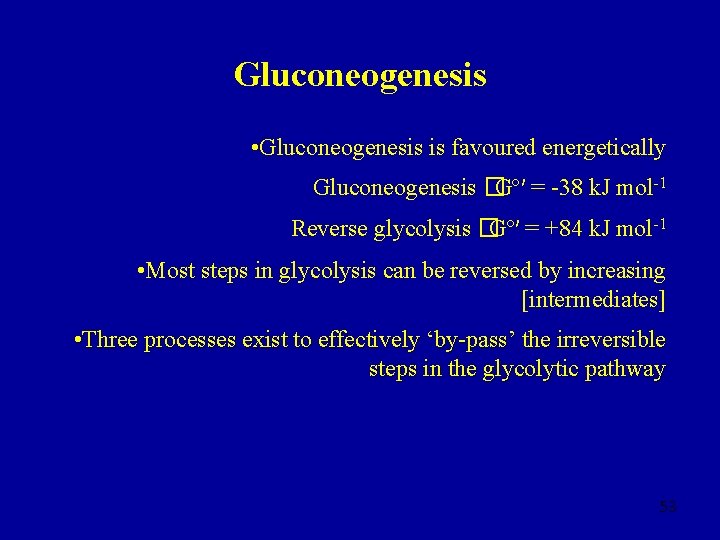
Gluconeogenesis • Gluconeogenesis is favoured energetically Gluconeogenesis �Gº′ = -38 k. J mol-1 Reverse glycolysis �Gº′ = +84 k. J mol-1 • Most steps in glycolysis can be reversed by increasing [intermediates] • Three processes exist to effectively ‘by-pass’ the irreversible steps in the glycolytic pathway 53

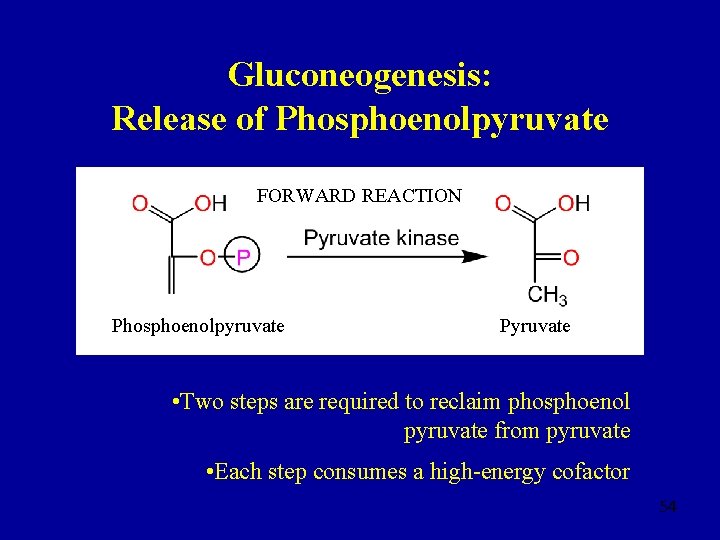
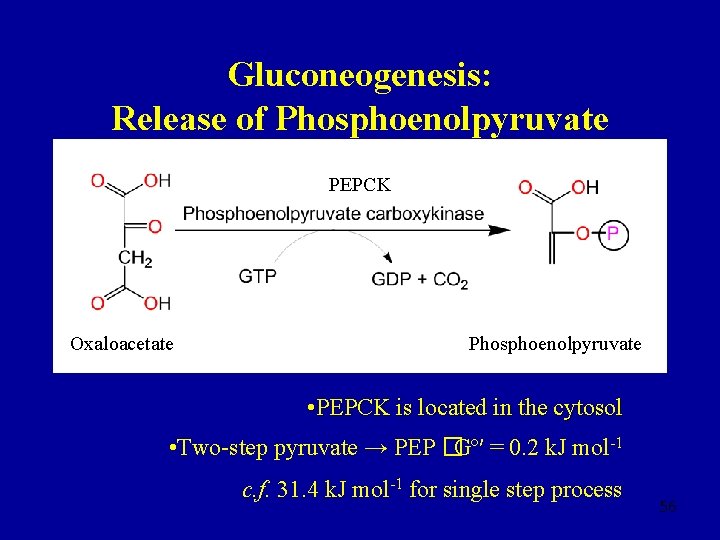
Gluconeogenesis: Release of Phosphoenolpyruvate FORWARD REACTION Phosphoenolpyruvate Pyruvate • Two steps are required to reclaim phosphoenol pyruvate from pyruvate • Each step consumes a high-energy cofactor 54

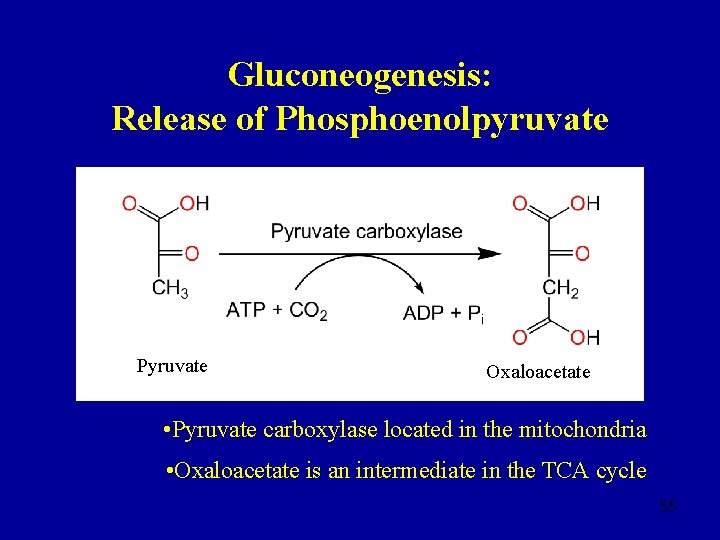
Gluconeogenesis: Release of Phosphoenolpyruvate Pyruvate Oxaloacetate • Pyruvate carboxylase located in the mitochondria • Oxaloacetate is an intermediate in the TCA cycle 55

Gluconeogenesis: Release of Phosphoenolpyruvate PEPCK Oxaloacetate Phosphoenolpyruvate • PEPCK is located in the cytosol • Two-step pyruvate → PEP �Gº′ = 0. 2 k. J mol-1 c. f. 31. 4 k. J mol-1 for single step process 56

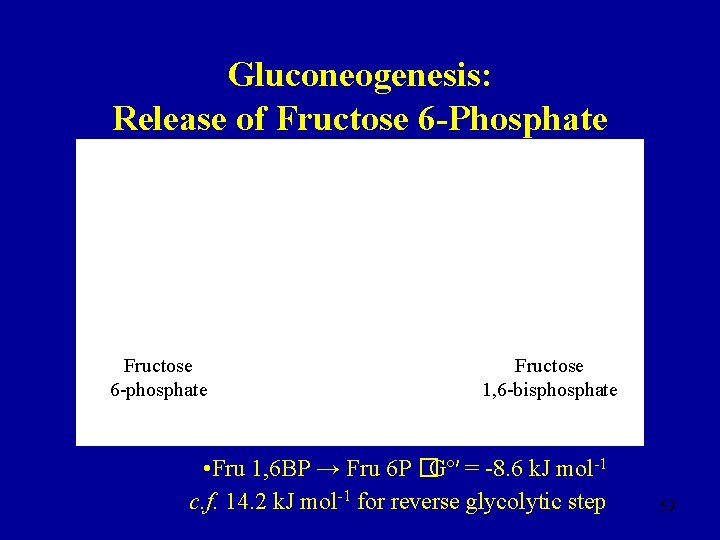
Gluconeogenesis: Release of Fructose 6 -Phosphate Fructose 6 -phosphate Fructose 1, 6 -bisphosphate • Fru 1, 6 BP → Fru 6 P �Gº′ = -8. 6 k. J mol-1 c. f. 14. 2 k. J mol-1 for reverse glycolytic step 57

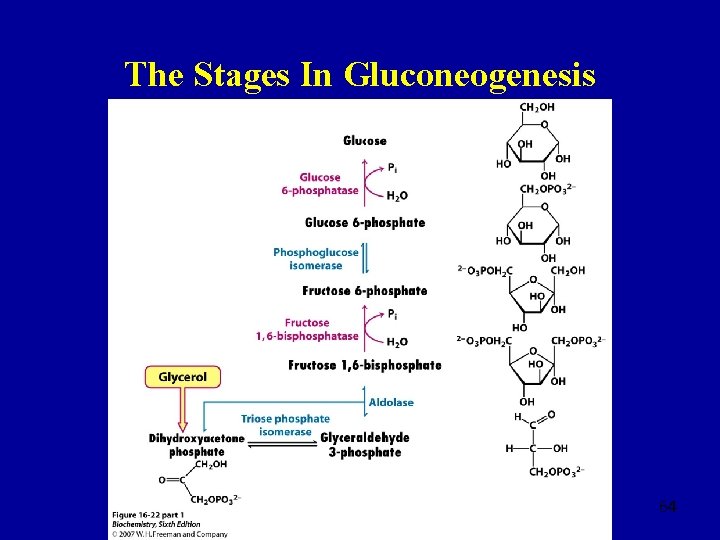
Gluconeogenesis: Release of Glucose by the Liver Glucose 6 -phosphate (e. g. from glycogen) • Glc 6 P → Glc takes place in ER 58

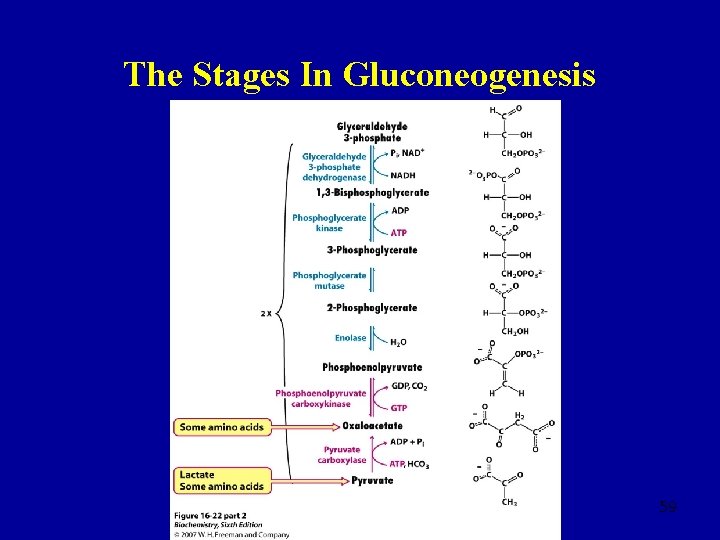
The Stages In Gluconeogenesis 59

Glycogen metabolism Blood glucose can be obtained from 3 primary sources: - Diet - Degradation of glycogen - Gluconeogenesis In the absence of a dietary source of glucose, glucose is rapidly released from liver glycogen, similarly, muscle glycogen is extensively degraded in working muscle. When glycogen stores are depleted, specific tissues synthesize glucose de novo, using amino acids from the body proteins as the primary source of carbons for the gluconeogenic pathway.

Glycogen metabolism - In the well-fed state, glycogen synthase is allosterically activated by glucose 6 -phosphate when it is present in elevated concentrations. In contrast, glycogen phophorylase is allosterically inhibited by glucose 6 phosphate, as well as by ATP, a high energy signal in the cell [ Note: in the liver, glucose also serves as an allosteric inhibitor of glycogen phosphorylase]. - Most of the glycogen molecule is degraded to glycose 1 -phosphate by the action of glycogen phosphorylase, the key enzyme in glycogen breakdown. The glycosidic linkage between C-1 of a terminal residue and C-4 of the adjacent one is split by orthophosphate to give glucose 1 phosphate that can converted into glucose 6 -phosphate.

- In muscle, phosphorylase is activated to generate glucose for use inside the cell as a fuel for contractile activity. - In contrast, liver phosphorylase is activated to liberate glucose for export to other organs, such as skeletal muscle and the brain. - Epinephrine and glucagon stimulate glycogen breakdown through specific 7 TM receptors. - Muscle is the primary target of epinephrine, whereas the liver is responsive to glucagon. Both signal molecules initiate a kinase cascade that lead to the activation of glycogen phosphorylase.

- In muscle, phosphorylase is activated to generate glucose for use inside the cell as a fuel for contractile activity. - In contrast, liver phosphorylase is activated to liberate glucose for export to other organs, such as skeletal muscle and the brain. - Epinephrine and glucagon stimulate glycogen breakdown through specific 7 TM receptors. - Muscle is the primary target of epinephrine, whereas the liver is responsive to glucagon. Both signal molecules initiate a kinase cascade that lead to the activation of glycogen phosphorylase.

The Stages In Gluconeogenesis 64

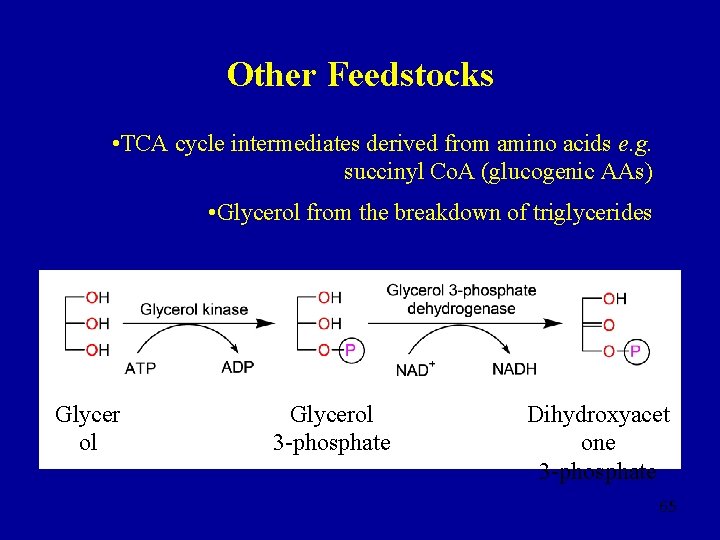
Other Feedstocks • TCA cycle intermediates derived from amino acids e. g. succinyl Co. A (glucogenic AAs) • Glycerol from the breakdown of triglycerides Glycer ol Glycerol 3 -phosphate Dihydroxyacet one 3 -phosphate 65

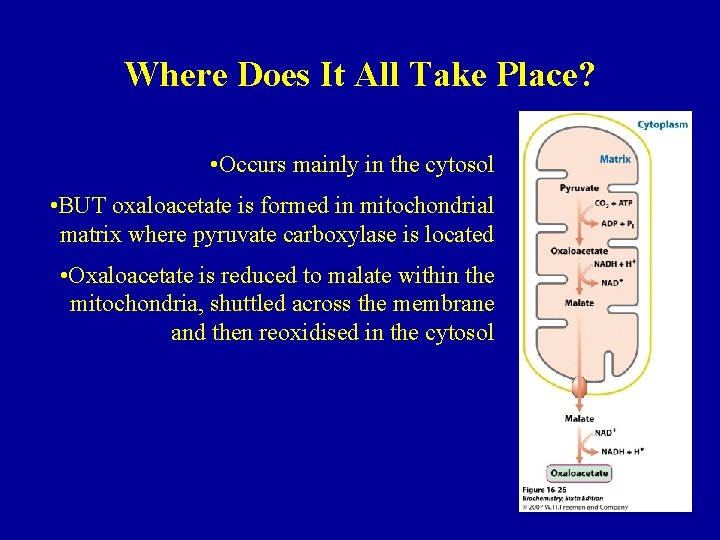
Where Does It All Take Place? • Occurs mainly in the cytosol • BUT oxaloacetate is formed in mitochondrial matrix where pyruvate carboxylase is located • Oxaloacetate is reduced to malate within the mitochondria, shuttled across the membrane and then reoxidised in the cytosol 66

Glycogen is synthesized and degraded by different pathways Glycogen is synthesized by different pathway from that of glycogen breakdown UDP-glucose, the activated intermediate in glycogen synthesis, is formed from glucose 1 -phosphate and UDP Glycogen synthase catalyzes the transfer of glucose from UDP-glucose to the C-4 hydroxyl group of a terminal residue in the growing glycogen molecule Synthesis is primed by glycogenin, an autoglycosylating protein that contains a covalently attached oligosaccharide unit on a specific tyrosine residue. A branching enzyme converts some of the alpha-1, 4 linkages into alpha-1, 6 linkages to increase the number of ends so that glycogen can be made and degraded more rapidly.

- In muscle, phosphorylase is activated to generate glucose for use inside the cell as a fuel for contractile activity. - In contrast, liver phosphorylase is activated to liberate glucose for export to other organs, such as skeletal muscle and the brain. - Epinephrine and glucagon stimulate glycogen breakdown through specific 7 TM receptors. - Muscle is the primary target of epinephrine, whereas the liver is responsive to glucagon. Both signal molecules initiate a kinase cascade that lead to the activation of glycogen phosphorylase.

Epinephrine inhibit this phosphatase by blocking its attachment to glycogen molecules and by turning on an inhibitor. insulin, in contrast, activates this phosphatase by triggering a cascade that phosphorylates that glycogen-targeting subunit of this enzyme. Hence, glycogen synthesis is decreased by epinephrine and increased by insulin. Glycogen synthase and phophorylase are also regulated by noncovalent allosteric interactions. Phosphorylase is a key part of the glucose-sensing system of liver cells. Glycogen metabolism exemplifies the power and precision of reversible phosphorylation in regulating biological processes.

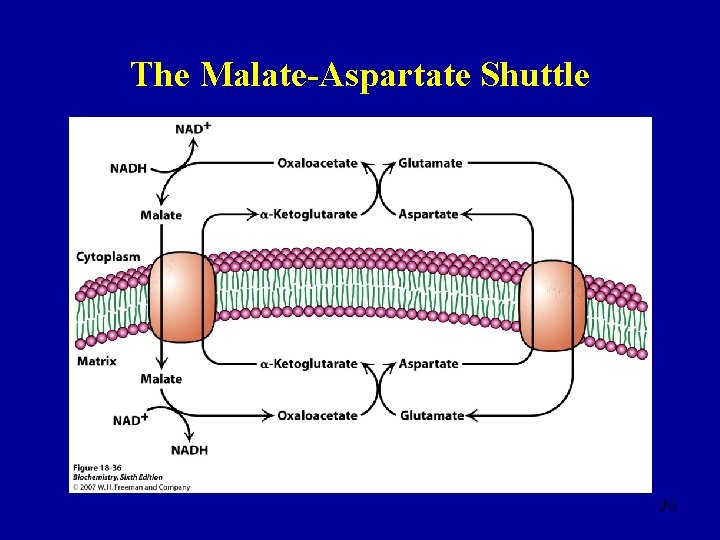
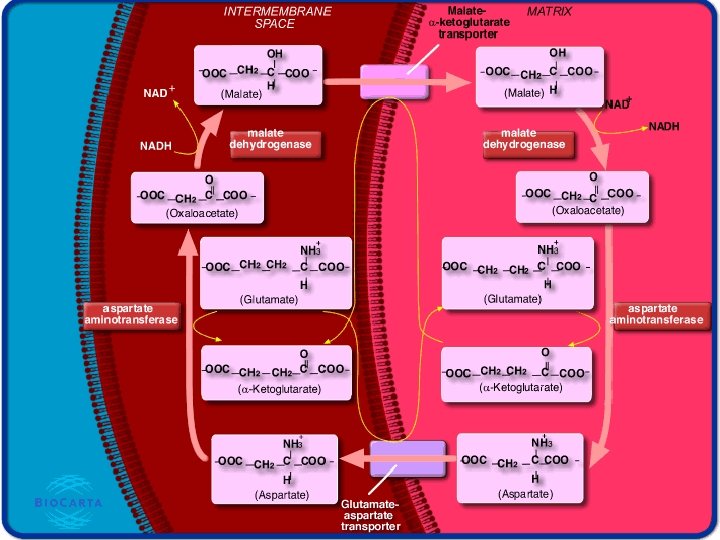
The Malate-Aspartate Shuttle 70

71

Regulation Of Gluconeogenesis 72

The Pathways Are Linked 73 Berg et al, ‘Biochemistry’, 5 th edition

Summary • Glycolysis has a number of reversible steps • The glycolytic process is driven by the three irreversible steps with most –ve �G • A variety of carbohydrates can take part in glycolysis though some require processing prior to entering the pathway • Glycolysis can be regulated at several points • Glycolysis and gluconeogenesis share a number of reversible steps but the three irreversible steps in the glycolytic pathway must be overcome 74