Glycolysis 5903 Glycolysis The conversion of glucose to














- Slides: 31

Glycolysis 5/9/03

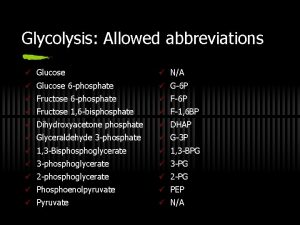
Glycolysis The conversion of glucose to pyruvate to yield 2 ATP molecules • 10 enzymatic steps • Chemical interconversion steps • Mechanisms of enzyme conversion and intermediates • Energetics of conversions • Mechanisms controlling the Flux of metabolites through the pathway


Historical perspective Winemaking and baking industries 1854 -1865 Louis Pasture established that microorganisms were responsible for fermentation. 1897 Eduard Buchner- cell free extracts carried out fermentation no “vital force” and put fermentation in the province of chemistry 1905 - 1910 Arthur Harden and William Young • inorganic phosphate was required ie. fructose-1, 6 bisphosphate • zymase and cozymase fractions can be separated by diaylsis

Inhibitors were used. Reagents are found that inhibit the production of pathway products, thereby causing the buildup of metabolites that can be identified as pathway intermediates. Fluoride- leads to the buildup of 3 -phosphoglycerate and 2 -phosphoglycerate 1940 Gustav Embden, Otto Meyerhof, and Jacob Parnas put the pathway together.

Pathway overview 1. Add phosphoryl groups to activate glucose. 2. Convert the phosphorylated intermediates into high energy phosphate compounds. 3. Couple the transfer of the phosphate to ADP to form ATP. Stage I A preparatory stage in which glucose is phosphorylated and cleaved to yield two molecules of glyceraldehyde-3 phosphate - uses two ATPs Stage II glyceraldehyde-3 -phosphate is converted to pyruvate with the concomitant generation of four ATPs-net profit is 2 ATPs per glucose. Glucose + 2 NAD+ + 2 ADP +2 Pi 2 NADH + 2 pyruvate + 2 ATP + 2 H 2 O + 4 H+

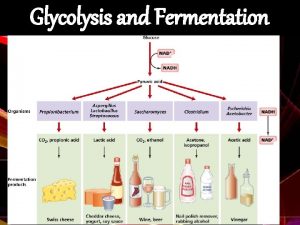
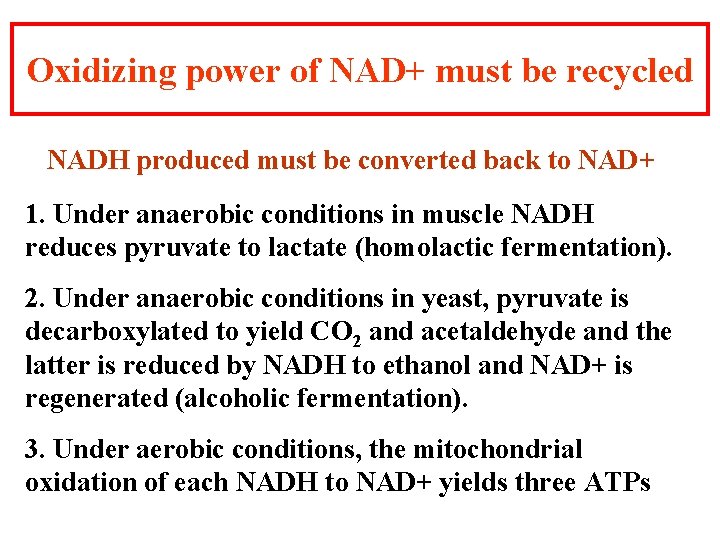
Oxidizing power of NAD+ must be recycled NADH produced must be converted back to NAD+ 1. Under anaerobic conditions in muscle NADH reduces pyruvate to lactate (homolactic fermentation). 2. Under anaerobic conditions in yeast, pyruvate is decarboxylated to yield CO 2 and acetaldehyde and the latter is reduced by NADH to ethanol and NAD+ is regenerated (alcoholic fermentation). 3. Under aerobic conditions, the mitochondrial oxidation of each NADH to NAD+ yields three ATPs


Hexokinase Mg++ + ATP Glucose + ADP + H+ Glucose-6 -phosphate Isozymes: Enzymes that catalyze the same reaction but are different in their kinetic behavior Tissue specific Glucokinase- Liver controls blood glucose levels. Hexokinase in muscle - allosteric inhibition by ATP Hexokinase in brain - NO allosteric inhibition by ATP

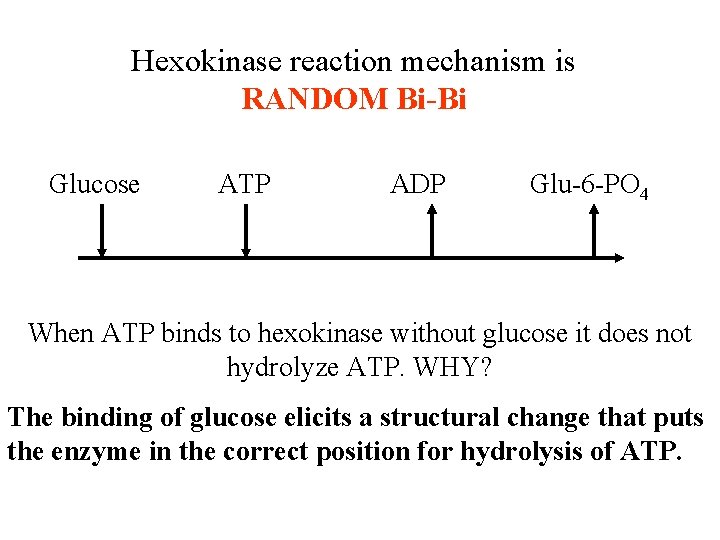
Hexokinase reaction mechanism is RANDOM Bi-Bi Glucose ATP ADP Glu-6 -PO 4 When ATP binds to hexokinase without glucose it does not hydrolyze ATP. WHY? The binding of glucose elicits a structural change that puts the enzyme in the correct position for hydrolysis of ATP.

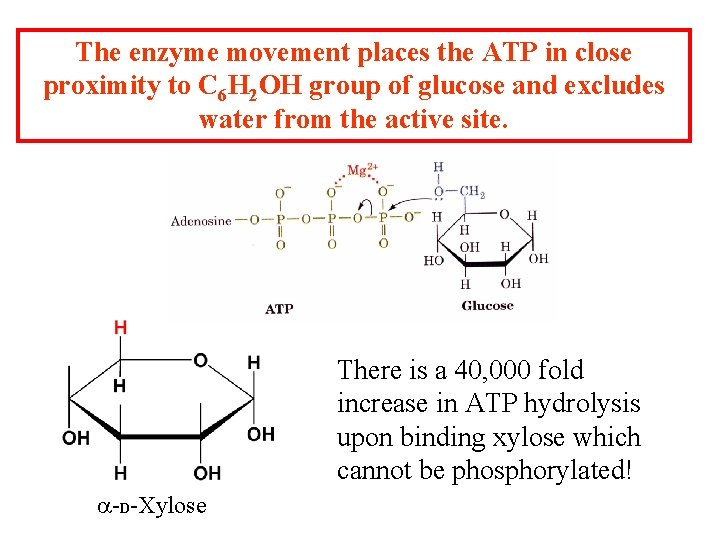
The enzyme movement places the ATP in close proximity to C 6 H 2 OH group of glucose and excludes water from the active site. There is a 40, 000 fold increase in ATP hydrolysis upon binding xylose which cannot be phosphorylated! a-D-Xylose

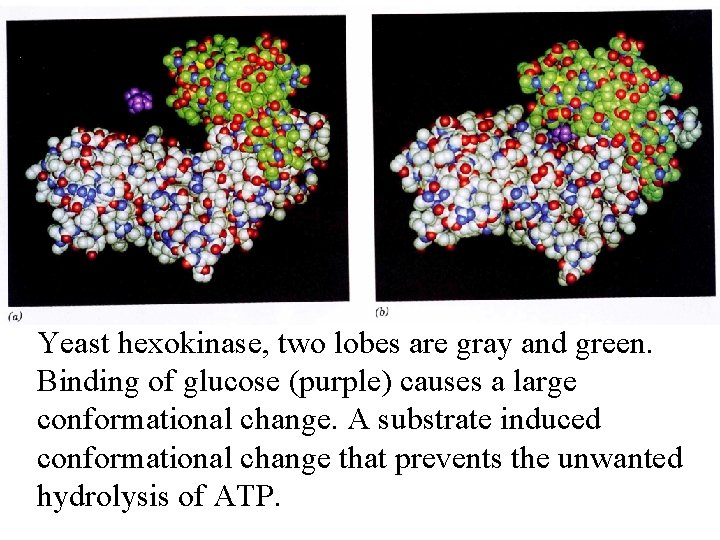
Yeast hexokinase, two lobes are gray and green. Binding of glucose (purple) causes a large conformational change. A substrate induced conformational change that prevents the unwanted hydrolysis of ATP.

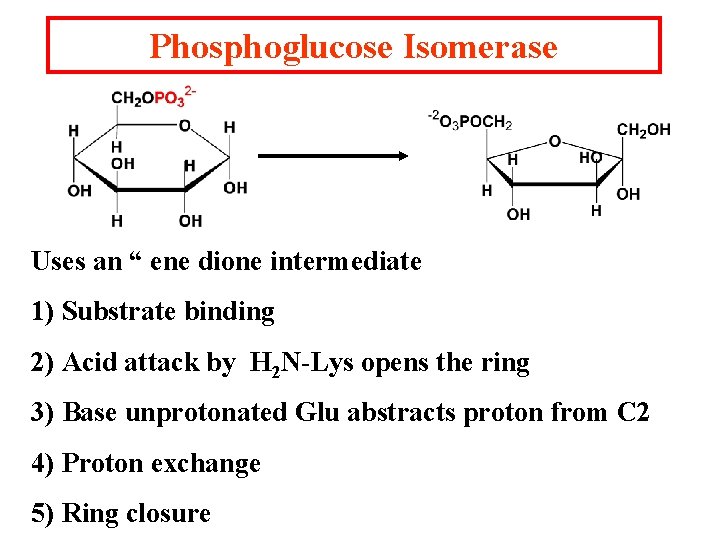
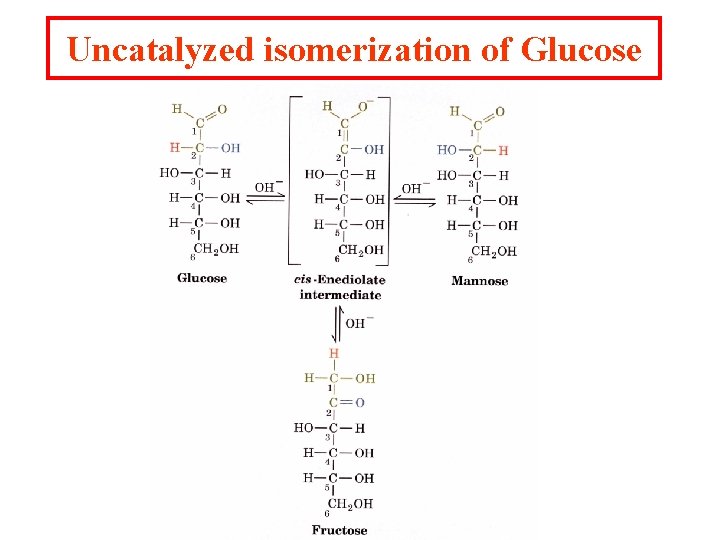
Phosphoglucose Isomerase Uses an “ ene dione intermediate 1) Substrate binding 2) Acid attack by H 2 N-Lys opens the ring 3) Base unprotonated Glu abstracts proton from C 2 4) Proton exchange 5) Ring closure

Uncatalyzed isomerization of Glucose


Phosphofructokinase + ATP Fructose-6 -PO 4 Mg++ + ADP Fructose-1, 6 -bisphosphate 1. ) Rate limiting step in glycolysis 2. ) Irreversible step, can not go the other way 3. ) The control point for glycolysis

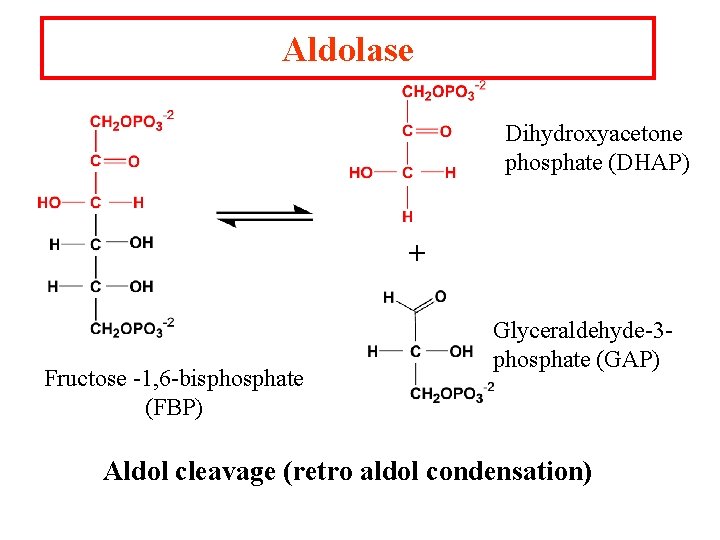
Aldolase Dihydroxyacetone phosphate (DHAP) + Fructose -1, 6 -bisphosphate (FBP) Glyceraldehyde-3 phosphate (GAP) Aldol cleavage (retro aldol condensation)

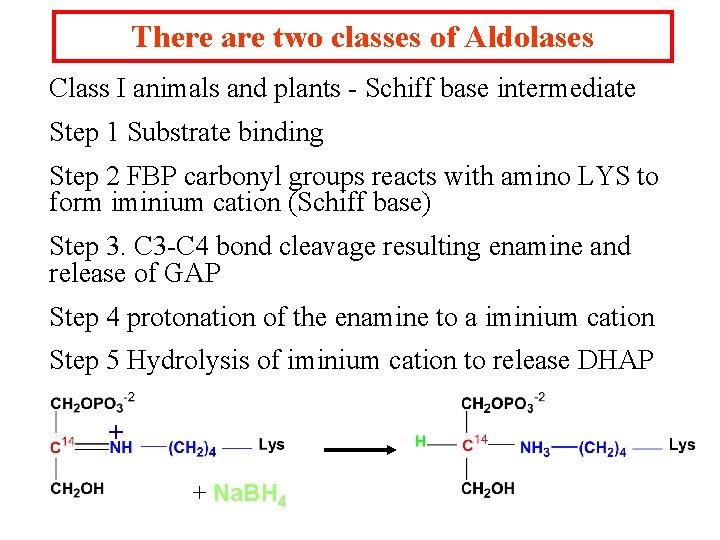
There are two classes of Aldolases Class I animals and plants - Schiff base intermediate Step 1 Substrate binding Step 2 FBP carbonyl groups reacts with amino LYS to form iminium cation (Schiff base) Step 3. C 3 -C 4 bond cleavage resulting enamine and release of GAP Step 4 protonation of the enamine to a iminium cation Step 5 Hydrolysis of iminium cation to release DHAP + + Na. BH 4

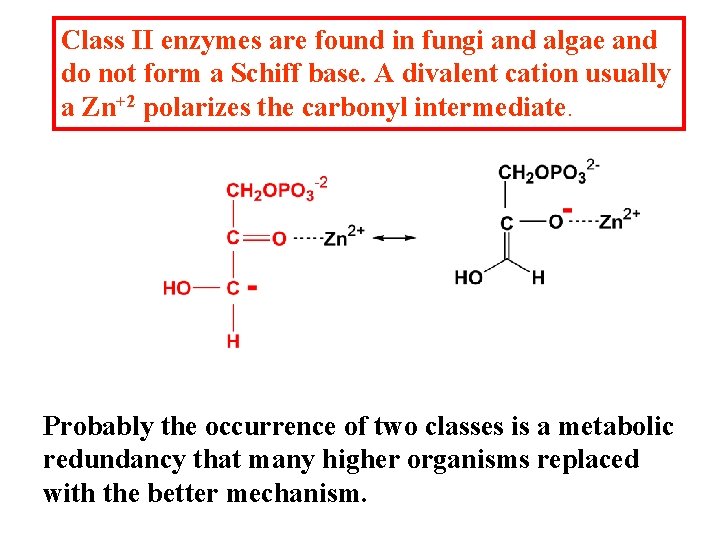
Class II enzymes are found in fungi and algae and do not form a Schiff base. A divalent cation usually a Zn+2 polarizes the carbonyl intermediate. Probably the occurrence of two classes is a metabolic redundancy that many higher organisms replaced with the better mechanism.


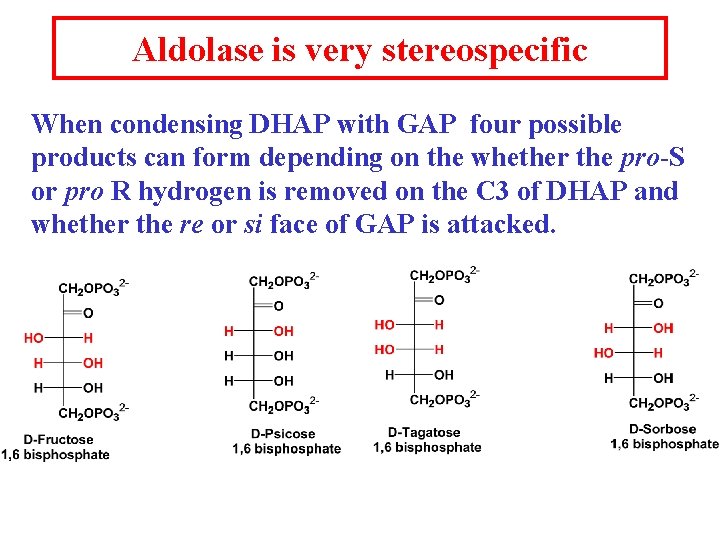
Aldolase is very stereospecific When condensing DHAP with GAP four possible products can form depending on the whether the pro-S or pro R hydrogen is removed on the C 3 of DHAP and whether the re or si face of GAP is attacked.

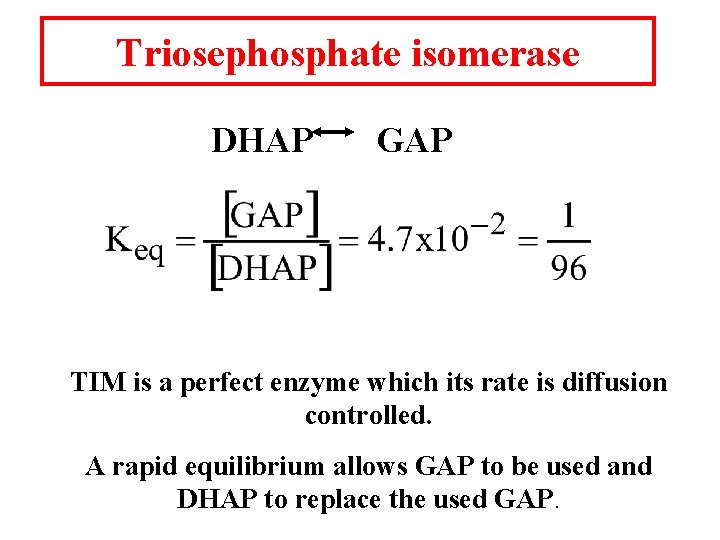
Triosephosphate isomerase DHAP GAP TIM is a perfect enzyme which its rate is diffusion controlled. A rapid equilibrium allows GAP to be used and DHAP to replace the used GAP.

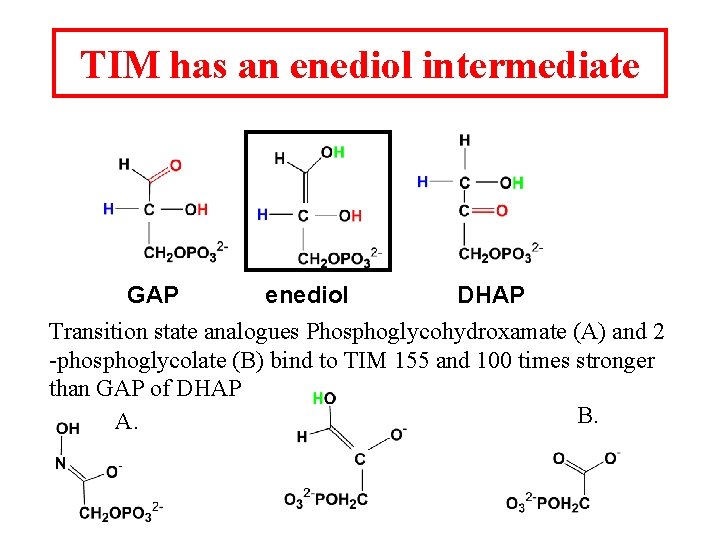
TIM has an enediol intermediate GAP enediol DHAP Transition state analogues Phosphoglycohydroxamate (A) and 2 -phosphoglycolate (B) bind to TIM 155 and 100 times stronger than GAP of DHAP B. A.

TIM has an extended “low barrier” hydrogen bond transition state Hydrogen bonds have unusually strong interactions and have lead to p. K of Glu 165 to shift from 4. 1 to 6. 5 and the p. K of


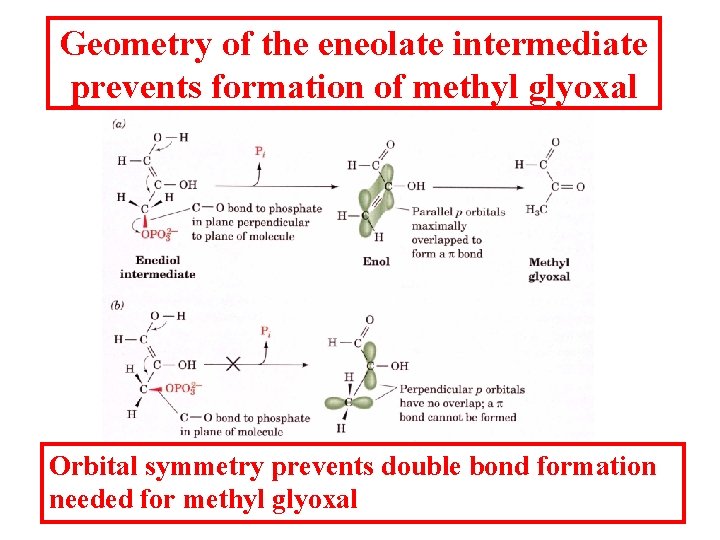
Geometry of the eneolate intermediate prevents formation of methyl glyoxal Orbital symmetry prevents double bond formation needed for methyl glyoxal

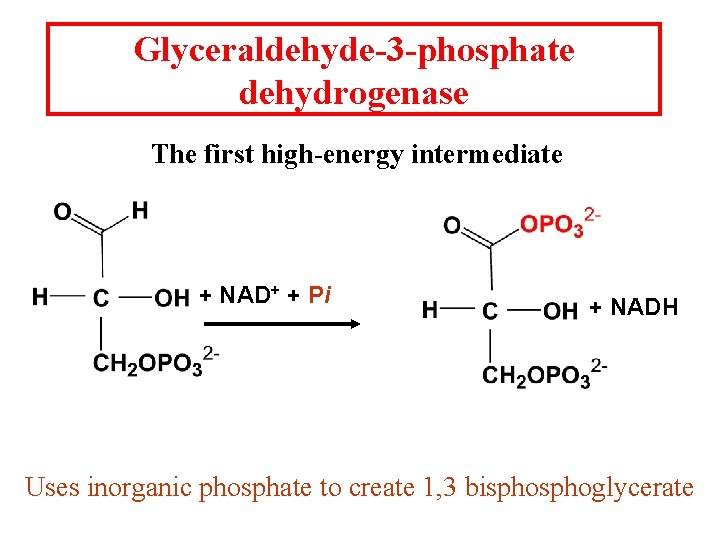
Glyceraldehyde-3 -phosphate dehydrogenase The first high-energy intermediate + NAD+ + Pi + NADH Uses inorganic phosphate to create 1, 3 bisphoglycerate

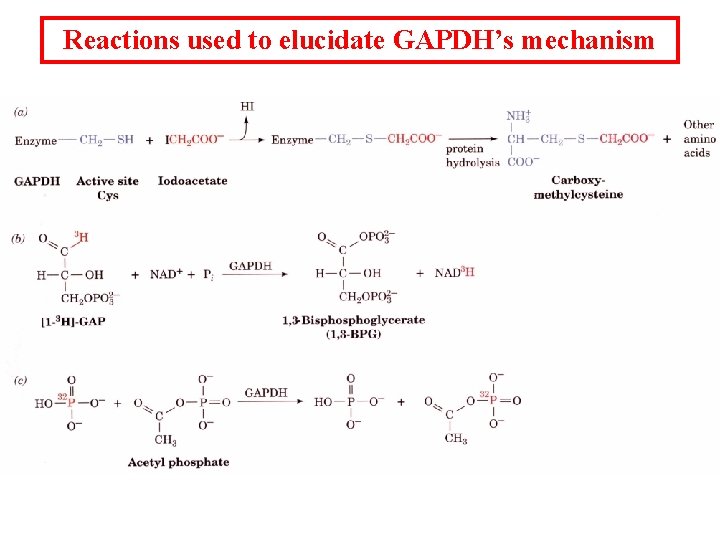
Reactions used to elucidate GAPDH’s mechanism


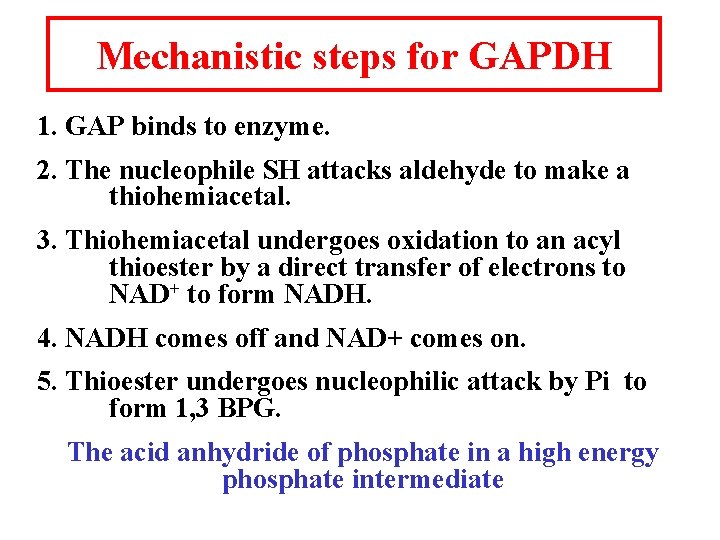
Mechanistic steps for GAPDH 1. GAP binds to enzyme. 2. The nucleophile SH attacks aldehyde to make a thiohemiacetal. 3. Thiohemiacetal undergoes oxidation to an acyl thioester by a direct transfer of electrons to NAD+ to form NADH. 4. NADH comes off and NAD+ comes on. 5. Thioester undergoes nucleophilic attack by Pi to form 1, 3 BPG. The acid anhydride of phosphate in a high energy phosphate intermediate

Arsenate uncouples phosphate formation 3 PG + GAP DH +
 Protein in csf
Protein in csf Difference between alpha and beta glucose
Difference between alpha and beta glucose Method of glucose estimation
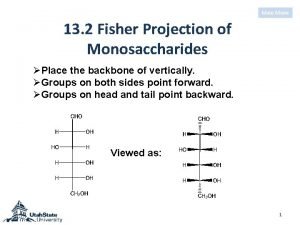
Method of glucose estimation Dihydroxyacetone fischer projection
Dihydroxyacetone fischer projection Công thức tiính động năng
Công thức tiính động năng Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể đại từ thay thế
đại từ thay thế Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Kể tên các môn thể thao
Kể tên các môn thể thao Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Phản ứng thế ankan
Phản ứng thế ankan Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Ng-html
Ng-html Sơ đồ cơ thể người
Sơ đồ cơ thể người So nguyen to
So nguyen to đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Chụp tư thế worms-breton
Chụp tư thế worms-breton ưu thế lai là gì
ưu thế lai là gì