glucose GLUTs glucose GLYCOGENESIS G 6 P GS

















- Slides: 29

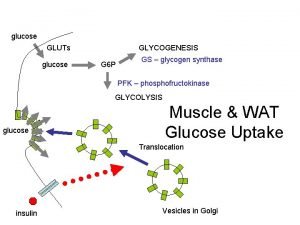
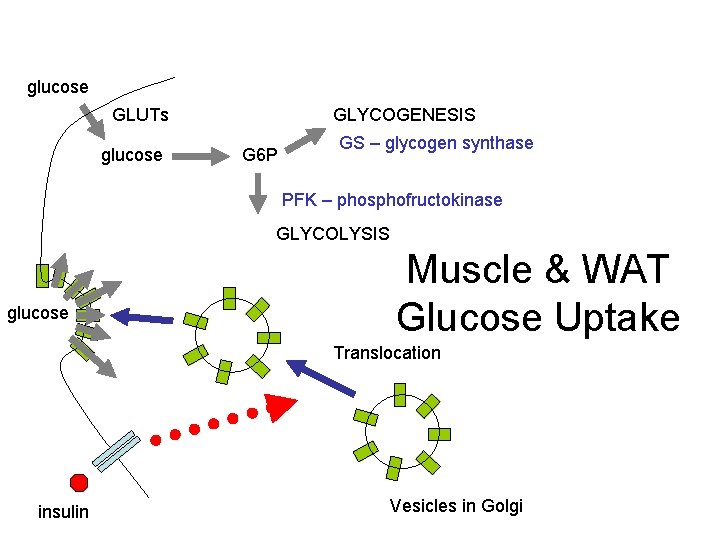
glucose GLUTs glucose GLYCOGENESIS G 6 P GS – glycogen synthase PFK – phosphofructokinase GLYCOLYSIS glucose Muscle & WAT Glucose Uptake Translocation insulin Vesicles in Golgi

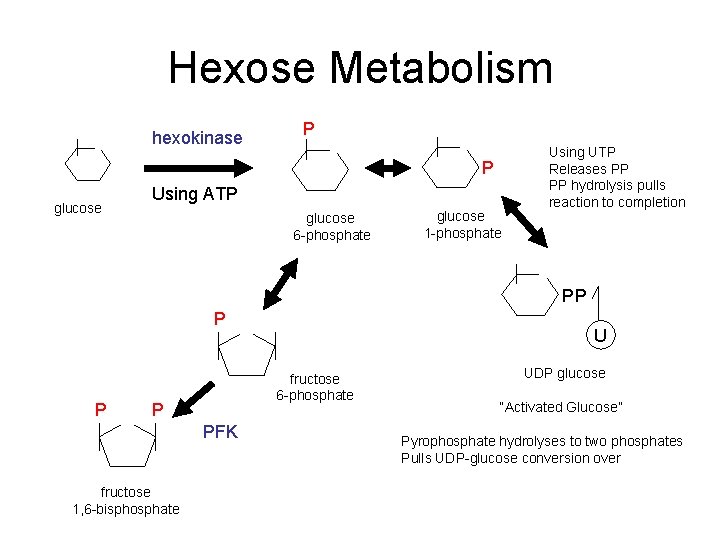
Hexose Metabolism hexokinase P P glucose Using ATP glucose 6 -phosphate glucose 1 -phosphate Using UTP Releases PP PP hydrolysis pulls reaction to completion PP P P fructose 6 -phosphate P PFK fructose 1, 6 -bisphosphate U UDP glucose “Activated Glucose” Pyrophosphate hydrolyses to two phosphates Pulls UDP-glucose conversion over

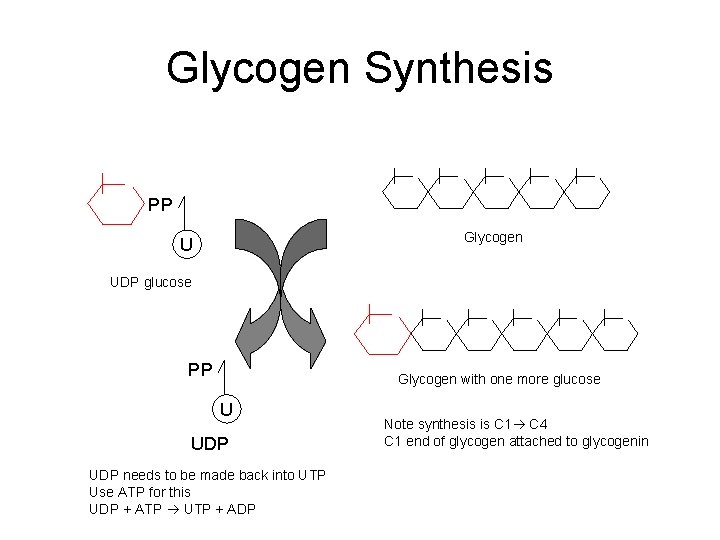
Glycogen Synthesis PP Glycogen U UDP glucose PP Glycogen with one more glucose U UDP needs to be made back into UTP Use ATP for this UDP + ATP UTP + ADP Note synthesis is C 1 C 4 C 1 end of glycogen attached to glycogenin

Glycogen Synthase • Catalyses the addition of ‘activated’ glucose onto an existing glycogen molecule – UDP-glucose + glycogenn UDP + glycogenn+1 • Regulated by reversible phosphorylation (covalent modification) – Active when dephosphorylated, inactive when phosphorylated • Phosphorylation happens on a serine residue – Dephosphorylation catalysed by phosphatases (specifically protein phosphatase I) – Phosphorylation catalysed by kinases (specifically glycogen synthase kinase) • Insulin stimulates PPI – And so causes GS to be dephosphorylated and active – So insulin effectively stimulates GS

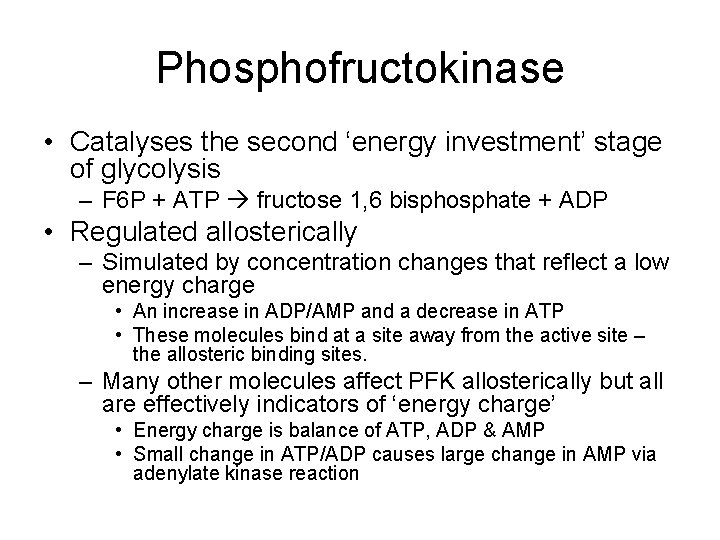
Phosphofructokinase • Catalyses the second ‘energy investment’ stage of glycolysis – F 6 P + ATP fructose 1, 6 bisphosphate + ADP • Regulated allosterically – Simulated by concentration changes that reflect a low energy charge • An increase in ADP/AMP and a decrease in ATP • These molecules bind at a site away from the active site – the allosteric binding sites. – Many other molecules affect PFK allosterically but all are effectively indicators of ‘energy charge’ • Energy charge is balance of ATP, ADP & AMP • Small change in ATP/ADP causes large change in AMP via adenylate kinase reaction

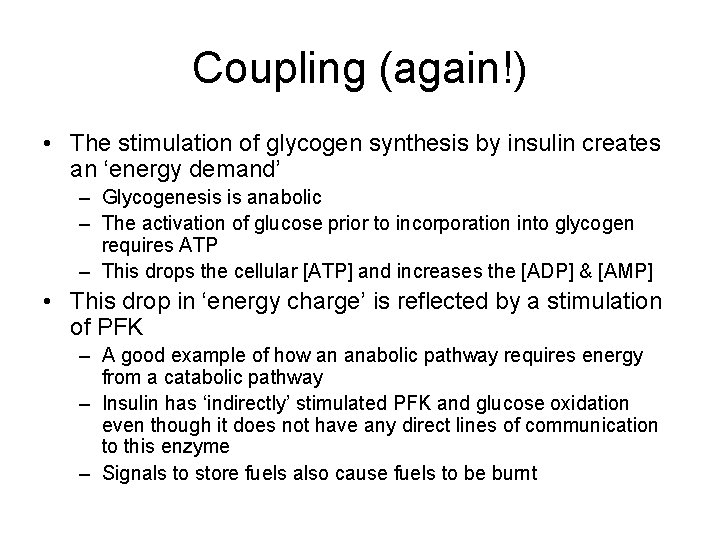
Coupling (again!) • The stimulation of glycogen synthesis by insulin creates an ‘energy demand’ – Glycogenesis is anabolic – The activation of glucose prior to incorporation into glycogen requires ATP – This drops the cellular [ATP] and increases the [ADP] & [AMP] • This drop in ‘energy charge’ is reflected by a stimulation of PFK – A good example of how an anabolic pathway requires energy from a catabolic pathway – Insulin has ‘indirectly’ stimulated PFK and glucose oxidation even though it does not have any direct lines of communication to this enzyme – Signals to store fuels also cause fuels to be burnt

Liver Glucose Uptake • GLUT-2 used to take up glucose from bloodstream – Very high activity and very abundant – [Glucose] blood = [Glucose] liver • Glucokinase – Rapidly converts G G 6 P – Not inhibited by build up of G 6 P – High Km (10 m. M) for glucose – not saturated by high levels of liver glucose – So [G 6 P] rapidly increases as blood [glucose] rises • G 6 P can stimulate inactive GS – Even phosphorylated GS – Glucose itself also stimulates the dephosphorylation of GS • Via a slightly complex process that involves other kinases and phosphatases which we needn’t go into right now

Glycogenesis • In liver – The “push” mechanism • Glycogenesis responds to blood glucose without the need of insulin • Although insulin WILL stimulate glycogenesis further • In muscle – [G 6 P] never gets high enough to stimulate GS • “Push” method doesn’t happen in muscle • More of a “pull’ as insulin stimulates GS • In both cases – 2 ATPs required for the incorporation of a glucose into glycogen chain • G G 6 P and UDP UTP – Branching enzyme needed to introduce a 1 6 branch points – Transfers a segment from one chain to another – Limit to the size of glycogen molecule • Branches become too crowded, even if they become progressively shorter • Glycogen synthase may need to interact with glycogenin to be fully active

A Tale of Two Kinases • Glucokinase (GK) – – – Only works on glucose High Km for glucose (~10 m. M) Not inhibited by G 6 P Only presents in liver, beta-cells Responsive to changes in [glucose] blood • Hexokinase (HK) – – – Works on any 6 C sugar Km for glucose ~0. 1 m. M Strongly inhibited by its product G 6 P Present in all other tissues If G 6 P is not used immediately, its build up and inhibits hexokinase – Easily saturated with glucose

Fructose Metabolism Glyceraldehyde fructokinase fructose Using ATP hexokinase P fructose 1 -phosphate Glyceraldehyde 3 phosphate Aldolase CH 2 OH B CHOH CHO CH 2 OP CHOH Triose Kinase CHO CH 2 OP P C=O CH 2 OH fructose 6 -phosphate Dihydroxyactone phosphate PFK ‘normal glycolysis’

Fructose Metabolism • Fructose entry into cells does not require insulin • In muscle, fructose just enters glycolysis – Or could be made into glycogen if insulin stimulus available! • F 6 P G 1 P UDP-glucose Glycogen • In liver, fructokinase traps fructose – FK produces F 1 P – FK is quite fast in comparison to the aldolase B that uses the F 1 P – F 1 P can build up – But more seriously producing ‘dead’ F 1 P traps phoshpate • FK reaction consumes ATP • Lack of phosphate akes new ATP synthesis difficult • ATP levels in liver fall – Even more serious in people with a deficiency in Aldolase B

Lipogenesis Overview glucose Fat ESTERIFICATION GLUT-4 No GS glucose X fatty acids G 6 P PPP Consumes reductant and ATP GLYCOLYSIS Produces reductant pyruvate LIPOGENESIS acetyl-Co. A pyruvate acetyl-Co. A PDH Key steps (eg, GLUT-4, PDH, lipogenesis) are stimulated when insulin binds to its receptor on the cell surface KREBS CYCLE CO 2 NADH release ultimately produces ATP

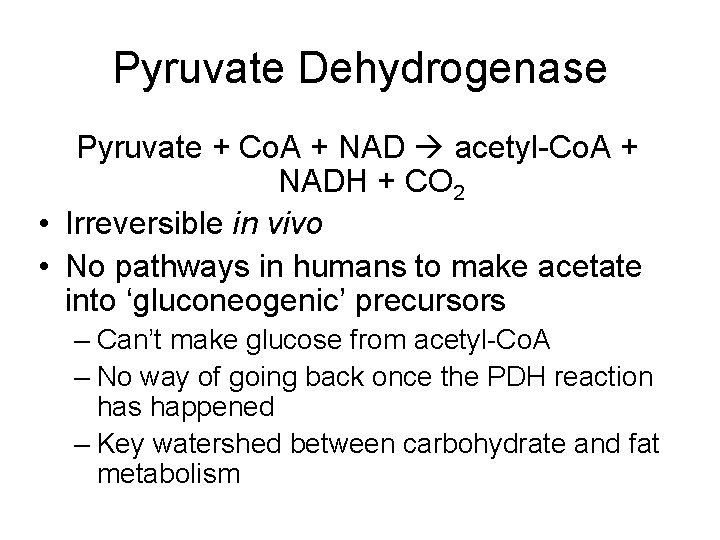
Pyruvate Dehydrogenase Pyruvate + Co. A + NAD acetyl-Co. A + NADH + CO 2 • Irreversible in vivo • No pathways in humans to make acetate into ‘gluconeogenic’ precursors – Can’t make glucose from acetyl-Co. A – No way of going back once the PDH reaction has happened – Key watershed between carbohydrate and fat metabolism

PDH Control • Regulated by reversible phosphorylation – Active when dephosphorylated • Inactivated by PDH kinase • Activated by PDH phosphatase – Insulin stimulates PDH phosphatase • Insulin thus stimulates dephosphorylation and activation of PDH

Fate of Acetyl-Co. A • Burnt in the Krebs Cycle – Carbon atoms fully oxidised to CO 2 – Lots of NADH produced to generate ATP • Lipogenesis – Moved out into the cytoplasm – Activated for fat synthesis • In both cases the first step is citrate formation – Condensation of acetyl-Co. A with oxaloacetate • Regenerates Coenzyme A – Transport or Oxidation • The ‘fate’ will depend on the need for energy (ATP/energy charge) and the stimulus driving lipogenesis

ATP-Citrate Lyase • Once in the cytoplasm, the citrate is cleaved – By ATP-Citrate Lyase (ACL) – Using Co. A to generate acetyl-Co. A and oxaloacetate • Reaction requires ATP ADP + phosphate • ACL is inhibited by hydroxy-citrate (OHCit) – OHCit is found in the Brindleberry • Sold as a fat synthesis inhibitor – Would we expect it to prevent the formation of fatty acids • And, if so, would that actually help us lose weight?

The Carrier • Oxaloacetate produced by ACL needs to return to the matrix – Otherwise the mitochondrial oxaloacetate pool becomes depleted – Remember, oxaloacetate is really just a ‘carrier’ of acetates • Both in the Krebs's cycle and in the transport of acetyl-Co. As into the cytoplasm – Oxaloacetate cannot cross the inner mitochondrial membrane • Some interesting inter-conversions occur to get it back in!

Acetyl-Co. A Carboxylase • Activates acetyl-Co. A and ‘primes’ it for lipogenesis • Unusual in that it ‘fixes’ carbon dioxide – In the form of bicarbonate – A carboxylation reaction Acetyl-Co. A + CO 2 malonyl-Co. A – Reaction requires ATP ADP + phosphate – Participation of the cofactor, biotin • Biotin is involved in other carboxylation reactions

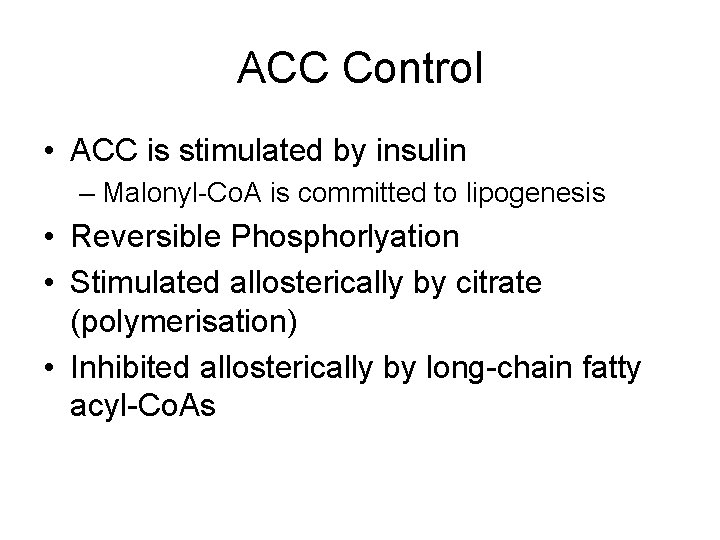
ACC Control • ACC is stimulated by insulin – Malonyl-Co. A is committed to lipogenesis • Reversible Phosphorlyation • Stimulated allosterically by citrate (polymerisation) • Inhibited allosterically by long-chain fatty acyl-Co. As

Malonyl-Co. A • Activated acetyl-Co. A – – Tagged and primed for lipogenesis But also a key regulator of fatty acid oxidation ACC is not only present in lipogenic tissues Also present in tissues that need to produce malonyl. Co. A in ‘regulatory’ amounts • Malonyl-Co. A inhibits carnitine acyl transferase I – An essential step in fatty acid oxidation – Only way of getting long chain fatty acyl-Co. As into the mitochondria

Malonyl-Co. A • So when ACC is active in, say, muscle – Malonyl-Co. A concentration rises – CPT-1 is inhibited – Fatty acid oxidation stops – Cell must use carbohydrate instead – Therefore insulin, by stimulating acetyl-Co. A carboxylase, encourages carbohydrate oxidation and inhibits fatty acid oxidation

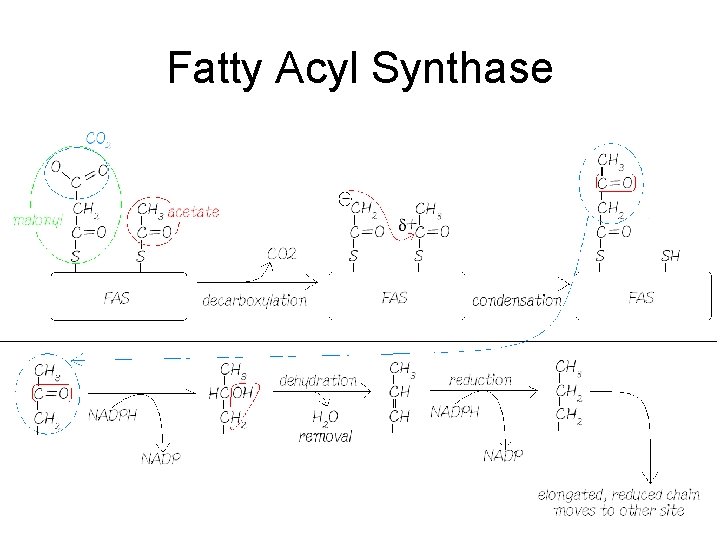
Fatty Acyl Synthase

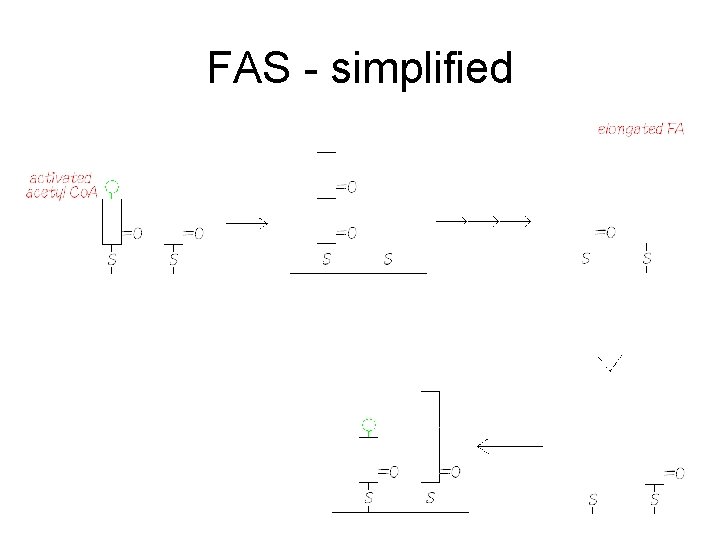
FAS - simplified

FAS • Fatty acyl synthase (FAS) is multi-functional – Lots of different enzyme activities in the complex – Can you count them all? • Bringing in acetyl and malonyl groups, catalysing the reaction between the decarboxylated malonyl and the growing fatty acid chain, the reduction/dehydration/reduction steps, moving the fatty acid to the right site and finally releasing it as FA-Co. A • Two free -SH groups on an ‘acyl-carring protein’ – Keeps the intermediates in exactly the right position for interaction with the right active sites – Each new 2 C unit is added onto the carboxy-end

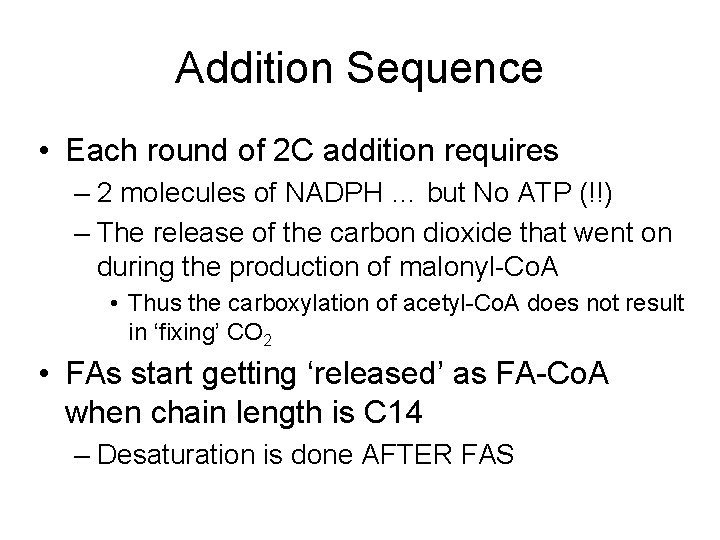
Addition Sequence • Each round of 2 C addition requires – 2 molecules of NADPH … but No ATP (!!) – The release of the carbon dioxide that went on during the production of malonyl-Co. A • Thus the carboxylation of acetyl-Co. A does not result in ‘fixing’ CO 2 • FAs start getting ‘released’ as FA-Co. A when chain length is C 14 – Desaturation is done AFTER FAS

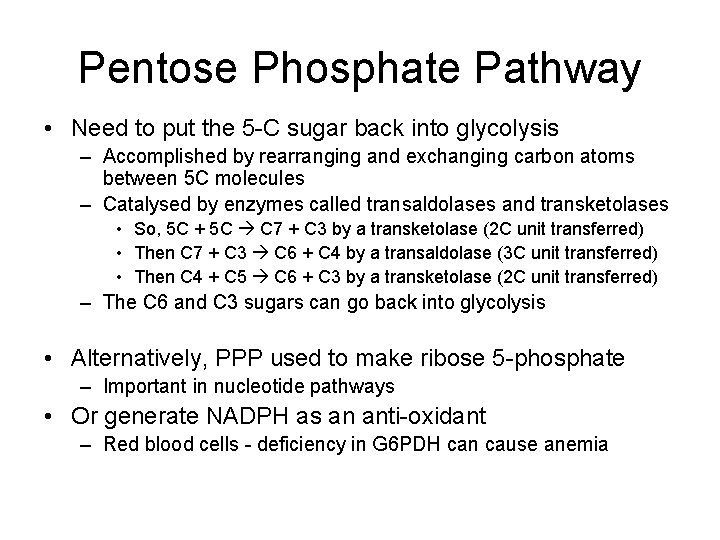
Pentose Phosphate Pathway • Provides NADPH for lipogenesis – NADPH - A form of NADH involved in anabolic reactions – Rate of NADPH production by PPP is proportional to demand for NADPH • Key regulatory enzyme is G 6 PDH – Glucose 6 -phosphate dehydrogenase G 6 P + NADP 6 -phosphogluconolactone + NADPH – The gluconolactone is further oxidised to give more NADPH • Decarboxylation to give a 5 -carbon sugar phosphate (ribulose 5 -phosphate)

Pentose Phosphate Pathway • Need to put the 5 -C sugar back into glycolysis – Accomplished by rearranging and exchanging carbon atoms between 5 C molecules – Catalysed by enzymes called transaldolases and transketolases • So, 5 C + 5 C C 7 + C 3 by a transketolase (2 C unit transferred) • Then C 7 + C 3 C 6 + C 4 by a transaldolase (3 C unit transferred) • Then C 4 + C 5 C 6 + C 3 by a transketolase (2 C unit transferred) – The C 6 and C 3 sugars can go back into glycolysis • Alternatively, PPP used to make ribose 5 -phosphate – Important in nucleotide pathways • Or generate NADPH as an anti-oxidant – Red blood cells - deficiency in G 6 PDH can cause anemia

Esterification • Formation of Fat • Glycerol needs to be glycerol 3 -phosphate – From reduction of glycolytic glyceraldehyde 3 -phosphate – Glycolysis important both for production of acetyl-Co. A and glycerol! • Esterification enzyme uses FA-Co. A – Not just FAs – FAs added one at a time • Both esterification enzyme and FAS are unregulated by insulin – Gene expression and protein synthesis • FAS is downregulated when lots of fat around – As in a Western diet!!

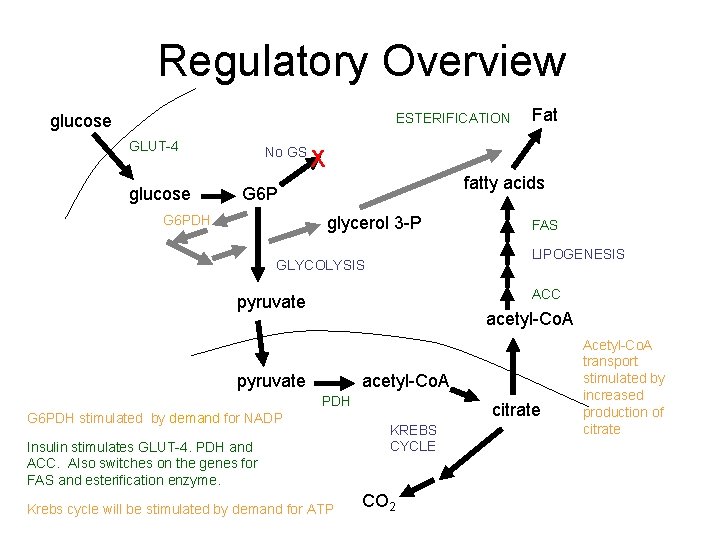
Regulatory Overview ESTERIFICATION glucose GLUT-4 glucose No GS X fatty acids G 6 PDH Fat glycerol 3 -P FAS LIPOGENESIS GLYCOLYSIS ACC pyruvate acetyl-Co. A PDH G 6 PDH stimulated by demand for NADP Insulin stimulates GLUT-4. PDH and ACC. Also switches on the genes for FAS and esterification enzyme. Krebs cycle will be stimulated by demand for ATP citrate KREBS CYCLE CO 2 Acetyl-Co. A transport stimulated by increased production of citrate
 Glycogenesis
Glycogenesis Glucose 1 phosphate
Glucose 1 phosphate Glycogenesis
Glycogenesis Structure of glycogen
Structure of glycogen Glycogen color
Glycogen color Glucose synthesis from non-carbohydrate sources
Glucose synthesis from non-carbohydrate sources Glucose tolerance test graph
Glucose tolerance test graph D glucose and l glucose
D glucose and l glucose Csf calculation
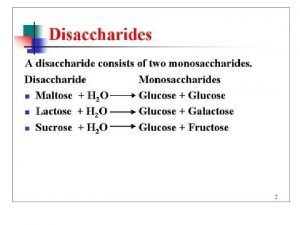
Csf calculation Maltose formation
Maltose formation Decreased glucose
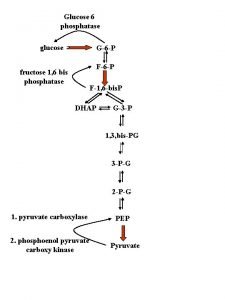
Decreased glucose Glucose 6 phosphatase
Glucose 6 phosphatase Foods that has glucose
Foods that has glucose Fates of glucose 6 phosphate
Fates of glucose 6 phosphate When glucose spills over into the urine, it has
When glucose spills over into the urine, it has Glucose intake
Glucose intake Cotransport transport actif
Cotransport transport actif Why is glucose polar
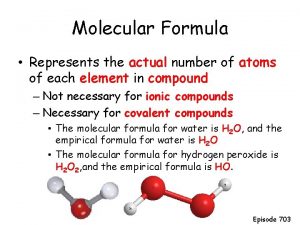
Why is glucose polar Define the molecular formula
Define the molecular formula Fasting plasma glucose concentration
Fasting plasma glucose concentration Glycosidic bond in glycogen
Glycosidic bond in glycogen Glucose+oxygen
Glucose+oxygen Glucose oxydase tp
Glucose oxydase tp Chapter 6 cell energy photosynthesis and respiration
Chapter 6 cell energy photosynthesis and respiration Glucose management indicator
Glucose management indicator Neogluconeogenesis
Neogluconeogenesis Methyl red test
Methyl red test Glucose reference range
Glucose reference range Photosystems
Photosystems Introduction of glucose
Introduction of glucose