GLP Principles Storage and retention of records and







- Slides: 8

GLP Principles Storage and retention of records and material. (Raw data and specimen)

RAW DATA • All original test facility records and documentation, or verified copies thereof, which are the result of the original observations and activities in a study. • Any laboratory worksheets, records, memoranda, notes, or exact copies thereof, that are the result of original observations and activities of a nonclinical laboratory study and are necessary for the reconstruction and evaluation of that study. • Raw data is a term that is used in both good manufacturing practice (GMP) and good laboratory practice (GLP) laboratories but it can create misunderstanding. • The records are therefore a great deal more than a list of figures. • All data generated during the conduct of a study should be identified and recorded directly, promptly, accurately, legibly and indelibly by the person entering the data, and be signed or initialled, and dated.

The raw data should include : “WHAT was done” • Describe procedures carried out and demonstrate that the instructions in the protocol were carried out, that relevant SOPs were followed and that the results of the observation or measurement were included. “HOW it was done” • Indicating that data were collected and recorded in accordance with the methods set out in the SOPs and protocol. “WHEN the work was performed” • Demonstrating that the timeline in the protocol was followed. This should be done by recording the date, and, if necessary, the time at which procedures were carried out. “WHO performed the work” • The data should clearly identify who was responsible for carrying out the procedure and recording the data. Where more than one person was involved in a procedure this should be recorded in the data and the responsibilities of each described.

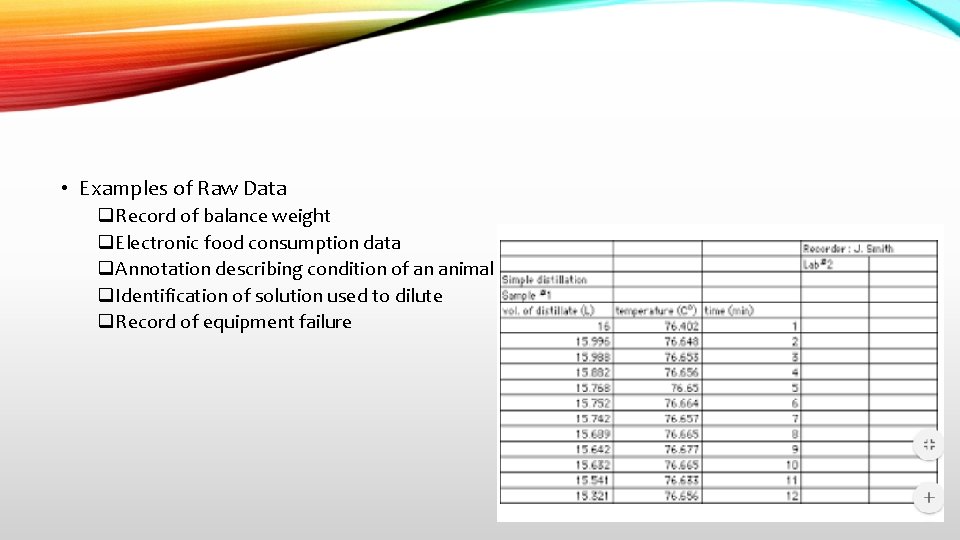
• Examples of Raw Data q. Record of balance weight q. Electronic food consumption data q. Annotation describing condition of an animal q. Identification of solution used to dilute q. Record of equipment failure

SPECIMEN • any material taken from a test system for examination or analysis • Each specimen should be clearly labelled with sufficient information for identification, enabling the test facility to confirm its contents. Ideally, labels should contain the following information: • • Test item name. Batch number. Expiry date. Storage conditions. Container number. Tare weight. Initial gross weight.

EXAMPLE OF SPECIMENS

REFERENCES

THE END THANK YOU