Glowing splint blown out Flaming splint on fire
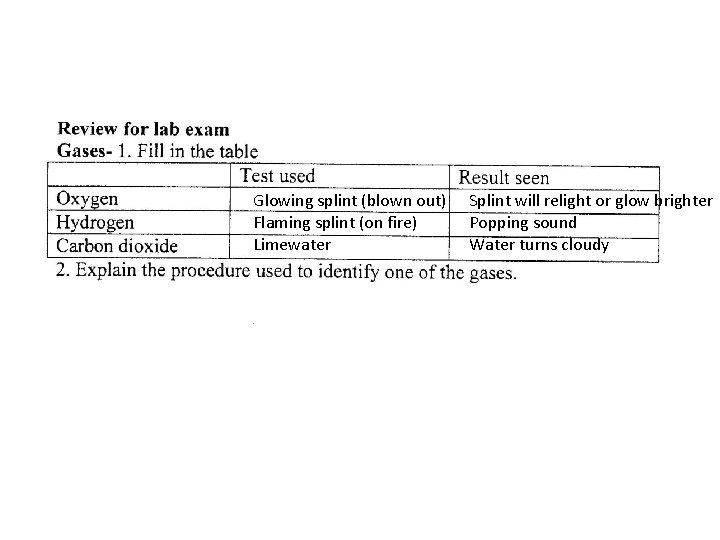
Glowing splint (blown out) Flaming splint (on fire) Limewater Splint will relight or glow brighter Popping sound Water turns cloudy
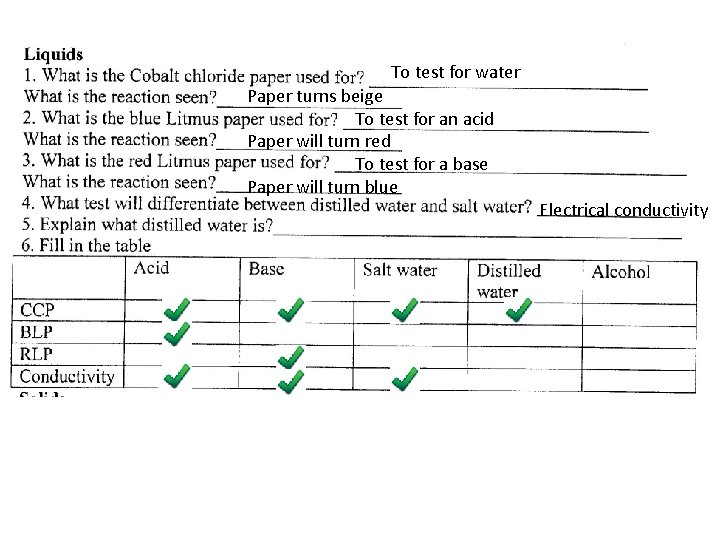
To test for water Paper turns beige To test for an acid Paper will turn red To test for a base Paper will turn blue Electrical conductivity

1. Add 10 m. L of water to the graduated cylinder 5. Find the mass on the balance 2. Add the solid 6. Calculate mass ÷ volume 3. Record the new volume in the graduated cylinder 4. Subtract the original volume from the new volume 1. 2. 3. 4. Measure the length, width and height Calculate volume L x W x H Find the mass on the balance Calculate density = mass ÷ volume Malleability: Can be bent without breaking (You might not be able to tell from a small sample) Metallic Luster: The solid is shiny
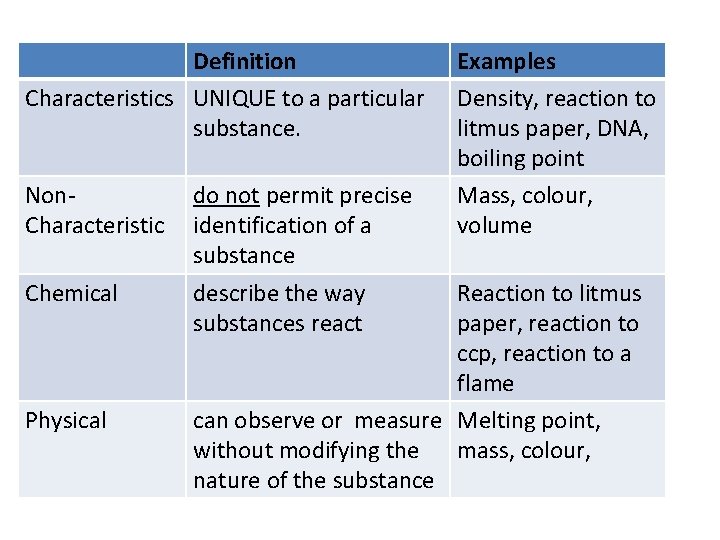
Definition Characteristics UNIQUE to a particular substance. Non. Characteristic Chemical Physical do not permit precise identification of a substance describe the way substances react Examples Density, reaction to litmus paper, DNA, boiling point Mass, colour, volume Reaction to litmus paper, reaction to ccp, reaction to a flame can observe or measure Melting point, without modifying the mass, colour, nature of the substance
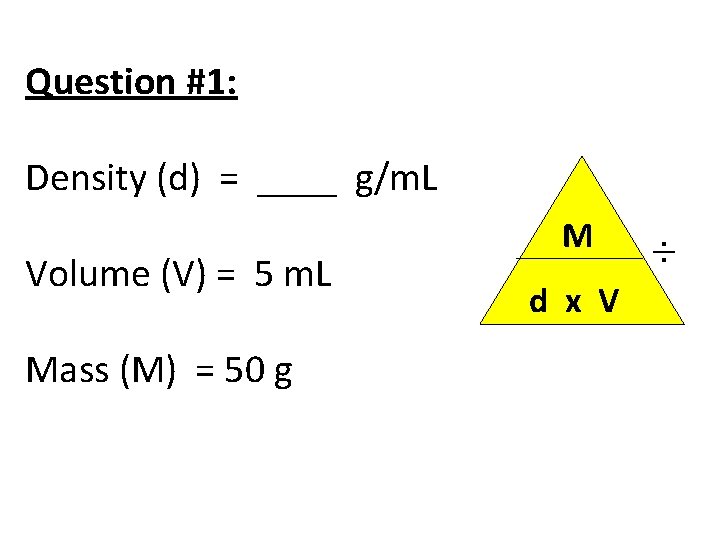
Question #1: Density (d) = ____ g/m. L Volume (V) = 5 m. L Mass (M) = 50 g M d x V ÷

Question #2: L = 2 cm W = 1 cm H = 5 cm Mass (M) = 70 g Density (d) = ____ g/m. L M d x V ÷
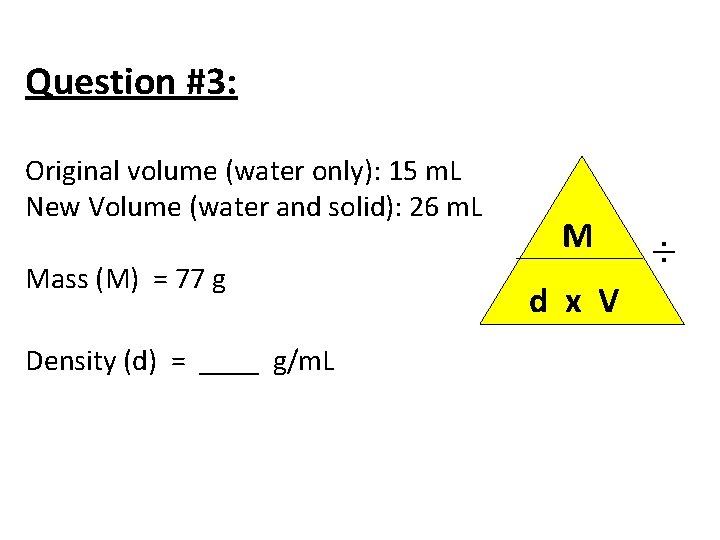
Question #3: Original volume (water only): 15 m. L New Volume (water and solid): 26 m. L Mass (M) = 77 g Density (d) = ____ g/m. L M d x V ÷

- Slides: 8