Global Metal Production 2001 Metal 106 Tonnes Zinc
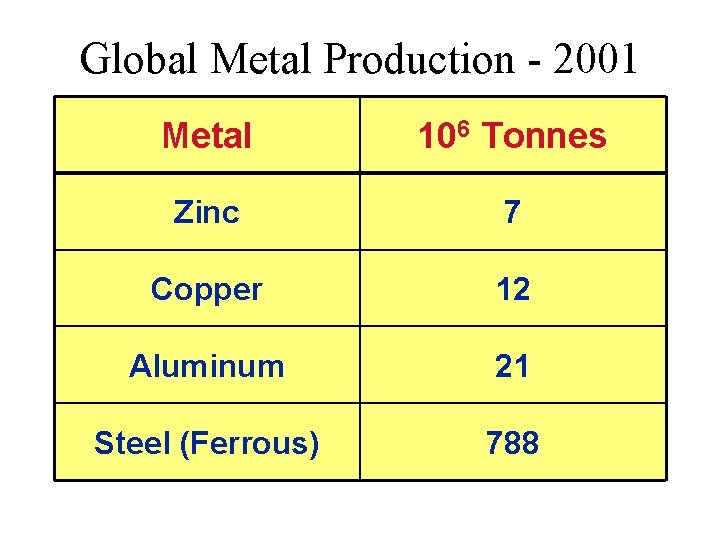
Global Metal Production - 2001 Metal 106 Tonnes Zinc 7 Copper 12 Aluminum 21 Steel (Ferrous) 788
Why Ferrous Dominates • Ore is cheap and abundant • Processing techniques are economical (extraction, refining, alloying, fabrication) • High strength • Very versatile metallurgy – a wide range of mechanical and physical properties can be achieved, and these can be tailored to the application
Ferrous Disadvantages • Low corrosion resistance – Oxidizes (rusts) easily – use, e. g. , titanium, brass instead • High Density: 7900 kg/m 3 (0. 29 lb/in 3) – Use, e. g. , aluminum, magnesium • High temperature strength could be better – Use Nickel or Cobalt Instead

BASIC DISTINCTION • BASIC DISTINCTION between FERROUS and NONferrous alloys: • Ferrous metals are ‘all-purpose’ alloys • Non-ferrous metals used for niche applications, where properties of ferrous metals are inadequate
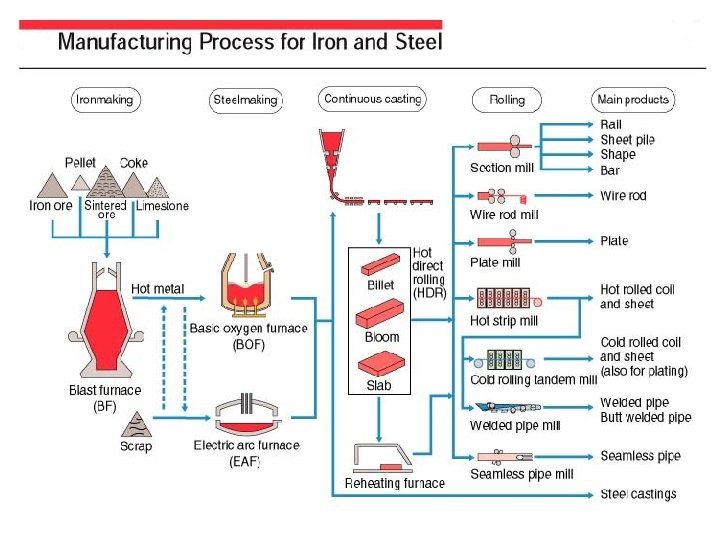
IRON ORE Among the elements composing the crust of the earth, iron exists in the largest quantity next to oxygen, silicon, and aluminum. Iron exists as natural ores in the form of oxides, and the estimated amount of ore deposits in the world is approximately 800 billion tons. Typical ores are hematite (Fe 2 O 3) and magnetite (Fe 3 O 4), having theoretical iron contents of 70% and 72%, respectively. The Fe content of practical ores is about 65% at maximum, and these ores include 2 -6% silica and 1 -3% alumina (Al 2 O 3). Representative sources of iron ores are found in mainland China, Brazil, Australia, the former USSR, America, India, Canada, South Africa, and elsewhere. The phosphorus and sulfur contents of ores differ greatly according to their origin
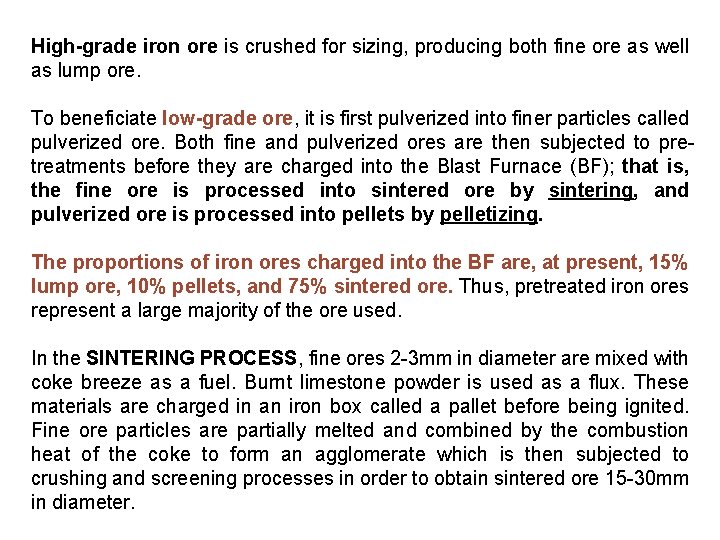
High-grade iron ore is crushed for sizing, producing both fine ore as well as lump ore. To beneficiate low-grade ore, it is first pulverized into finer particles called pulverized ore. Both fine and pulverized ores are then subjected to pretreatments before they are charged into the Blast Furnace (BF); that is, the fine ore is processed into sintered ore by sintering, and pulverized ore is processed into pellets by pelletizing. The proportions of iron ores charged into the BF are, at present, 15% lump ore, 10% pellets, and 75% sintered ore. Thus, pretreated iron ores represent a large majority of the ore used. In the SINTERING PROCESS, fine ores 2 -3 mm in diameter are mixed with coke breeze as a fuel. Burnt limestone powder is used as a flux. These materials are charged in an iron box called a pallet before being ignited. Fine ore particles are partially melted and combined by the combustion heat of the coke to form an agglomerate which is then subjected to crushing and screening processes in order to obtain sintered ore 15 -30 mm in diameter.

PELLETIZING is a process that involves mixing very finely ground particles of ore of less than 200 mesh with fluxing materials such as limestone and dolomite and then shaping them into balls 10 -15 mm in diameter by a pelletizer, and hardening the balls by firing with heavy oil and/or coal as a fuel. Cold-bond pellets are also produced by pelletizing, and do not require firing. At present, small-scale equipment for producing cold-bond pellets is in operation mainly to treat the dust collected in steel works. This technology offers great promise for the future in terms of energy-saving and reducing environmental pollution. Compared with sintered ore, pellets have a higher iron- and a lower gang-content, and pelletizing is suitable for treating the very fine ore that will predominate in the future. However, pellets have the disadvantages that more fossil fuel is consumed during pelletizing.
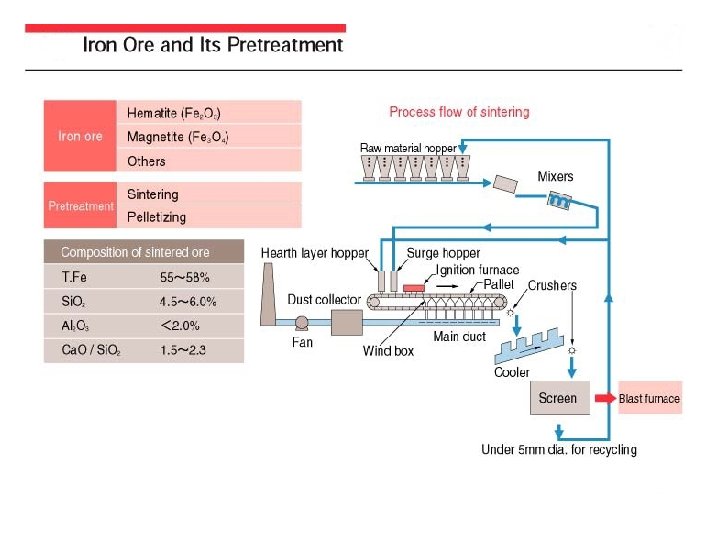
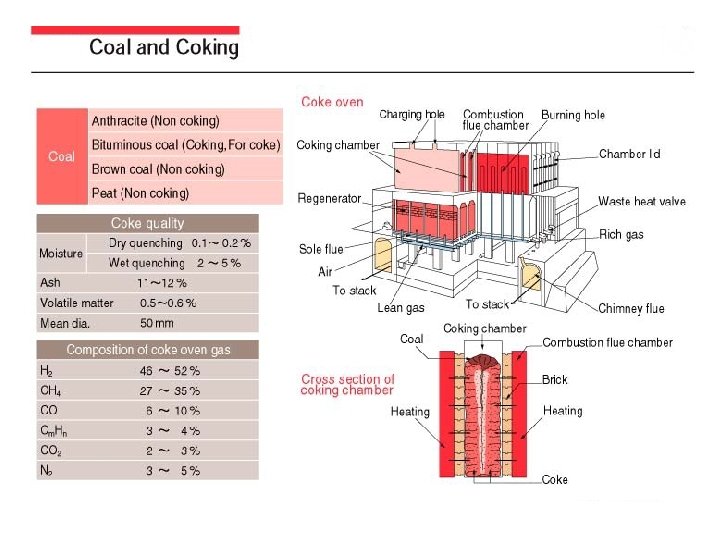

In the smelting process for iron and steel, coke serves as the source of carbon, which works as a reducing agent when reducing iron ore in the BF. At the same time, coke acts as the heat source for heating and melting the charged materials. Coke is made by baking coal in a coke oven. Coal is classified into the four grades shown in the figure, anthracite being the highest grade. Typical types are bituminous coal and brown coal. Bituminous coal exists in the largest quantities, having estimated reserves worldwide of approximately 7 trillion (trillion=1012)tons, with confirmed reserves of approximately 2 trillion tons. The coke used in the BF must have a high carbon content and low ash and sulfur contents, and must have an appropriate porosity as well as good strength to ensure that it gives good reactivity and does not pulverize to choke the gas flow in the BF even at high temperatures.

Cokes that meet these requirements are derived from bituminous coals that combine good coking properties with low ash and sulfur contents. In the coke oven, the raw coal obtained by crushing and blending is charged into the coke chamber, where it is then baked (carbonized) by indirect heating at 1, 200 -1, 300 C for 14 -18 hours to form coke that contains about 90% fixed carbon. The coking process also produces such by-products as gas, coal tar, and pitch which can be refined and treated into useful secondary products such as fuel gas, pure hydrogen gas, chemical products such as benzene, toluene, xylene, naphthalene, dye, and carbon fibers. The life of a coke oven is about 40 years. Technical developments are being made to provide new technology to (i) produce coke (ii) establish a cokeless iron making process both of which will make it possible to select raw coal materials more freely and will cause less environmental pollution.
- Slides: 12