Global Harmonisation Task Force and its SG 2

Global Harmonisation Task Force and its SG 2: An Overview Dr. Ekkehard Stösslein Federal Institute for Drugs and Medical Devices Tel. : +49 -228 -207 5384; Fax: +49 -228 -207 -5300 e. stoesslein@bfarm. de Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Content of the Presentation • Who is GHTF • Mission and purpose of the SG 2 • Members of the SG 2 • Major achievements Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Who is GHTF • The Global Harmonization Task Force (GHTF) is a voluntary group of representatives from national medical device regulatory authorities and the regulated industry. • comprises representatives from five founding members grouped into three geographical areas: Europe (EU) Asia-Pacific (Japan and Australia) North America (Canada and USA) each of which actively regulates medical devices using their own unique regulatory framework Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Purpose of GHTF • The purpose of the GHTF is to encourage convergence in regulatory practices related to ensuring the safety, effectiveness / performance and quality of medical devices, promoting technological innovation and facilitating international trade, and the primary way in which this is accomplished is via the publication and dissemination of harmonized guidance documents on basic regulatory practices. • The GHTF also serves as an information exchange forum through which countries with medical device regulatory systems under development can benefit from the experience of those with existing systems and/or pattern their practices upon those of GHTF founding members. Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

The 5 Study Groups (SG) • 5 study groups are dealing with – SG 1: Essential Principles of Safety & Performance of Medical Devices (labelling, classification, role of standards, technical documentation) – SG 2: Post Market Surveillance and Vigilance (who should what when report to whom) – SG 3: Quality System Requirements (design-, risk managemant control, process validation) – SG 4: Auditing (Language, Training, Regulatory Auditing Strategy) – SG 5: Clinical Investigations (requirements, definitions, documentation) See www. ghtf. org Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Members of the SG 2 • Europe • National Competent Authority (NCA) of UK, Switzerland Germany plus EU-Commission • Industry: European Medical Technology Industry Association (Eucomed), European Federation of Precision Mechanical and Optical Industries (Eurom VI) • North America • FDA and Health Canada (NCA) • Advanced Medical Technology Association (Adva. Med) and National Electrical Manufacturers Association (NEMA) Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Members of the SG 2 • Australia • Therapeutic Goods Agency (NCA) • Medical Industry Association of Australia • Japanese Ministry of Health, Labor and Welfare (NCA) • Japan Federation of Medical Devices Associations (Industry) In total: 8 regulators and 7 industry representatives Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Mission (SG 2 N 12 R 8) The purpose of a vigilance and postmarket surveillance system is to improve the protection of the health and safety of patients, users and others by reducing the likelihood of the same type of adverse incident being repeated in different places at different times. Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Mission (SG 2 N 12 R 8) The purpose of a vigilance and postmarket surveillance system is to improve the protection of the health and safety of patients, users and others by reducing the likelihood of the same type of adverse incident being repeated in different places at different times. This is to be achieved by the evaluation of reported incidents, and where appropriate, dissemination of information which could be used to prevent such repetitions, or to alleviate the consequences of such repetitions. Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Purpose (SG 2 N 12 R 8) This study group will define requirements for a common medical devices vigilance system and provide an international protocol to define and facilitate the transmission of vigilance information on a global basis. Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Major Tasks (SG 2 N 12 R 8) • Definitions • Who does the initial reporting of events (physicians, other clinical staff, owner/operator of device, patients, distributor, manufacturers)? • To whom are adverse events to report? • What information is when to report? • Who investigates the event with what means (failure analyses, depth depending on outcome? • Information excange between authorities Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Major Results • Global Guidance for Adverse Event Reporting (who reports what in what time frame) => central document of the group • National Competent Authority Report Exchange (criteria and what information is how to be exchanged) • Application Requirements for Participation in National Competent Authority Report Exchange (what criteria must be fulfilled to be a member of the exchange) • Harmonizing of the content of Field Safety Corrective Actions and Advisory notices • NO CONSENSUS: To whom to report (place of incident only or to multiple CAs) Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein
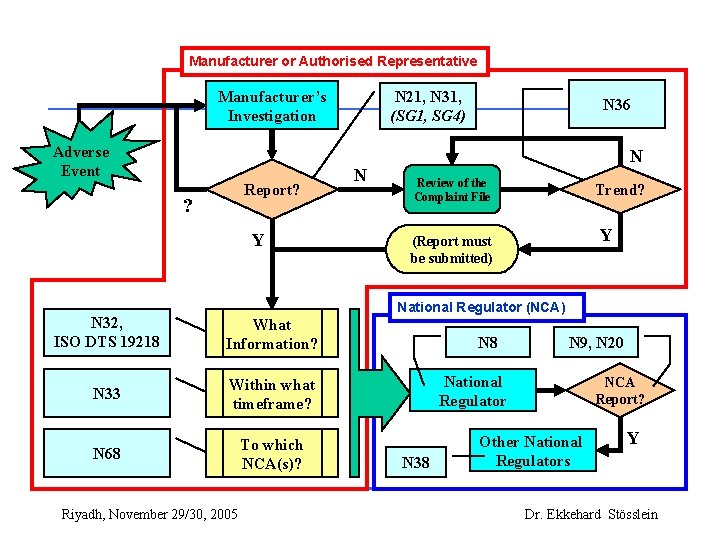
Manufacturer or Authorised Representative Manufacturer’s Investigation Adverse Event ? Report? Y N 21, N 31, (SG 1, SG 4) N N 36 N Review of the Complaint File Trend? Y (Report must be submitted) National Regulator (NCA) N 32, ISO DTS 19218 What Information? N 33 Within what timeframe? N 68 To which NCA(s)? N 8 N 9, N 20 National Regulator N 38 Other National Regulators NCA Report? Y Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein

Any Questions ? Riyadh, November 29/30, 2005 Dr. Ekkehard Stösslein
- Slides: 14