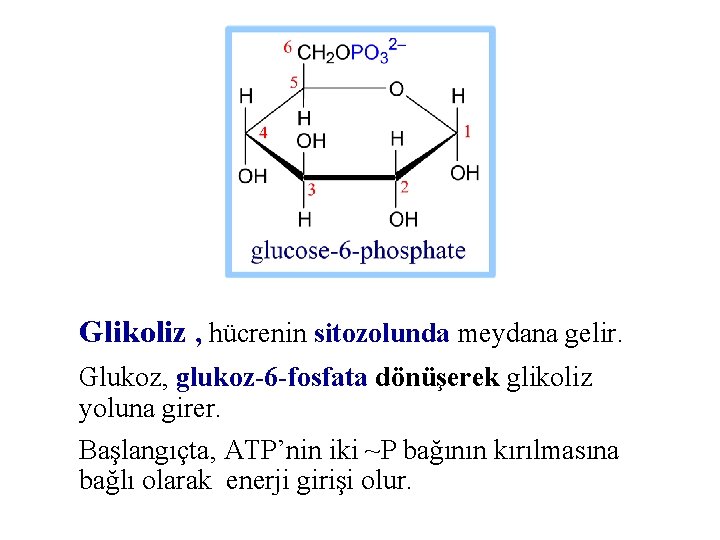
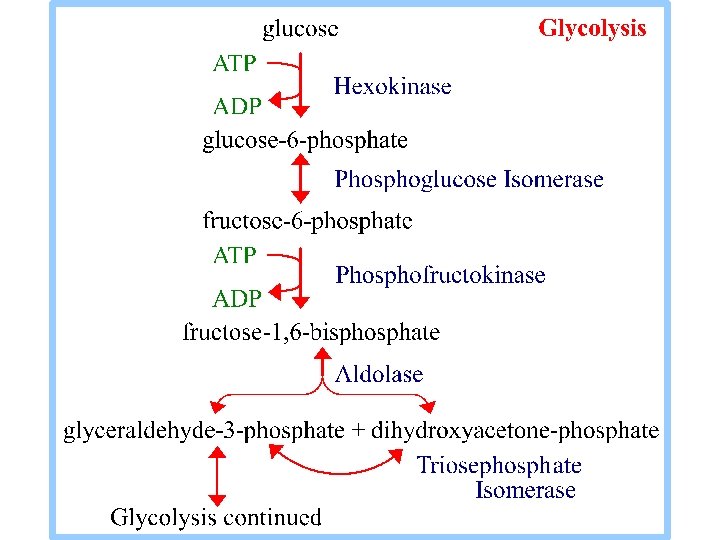
Glikoliz Glikoliz hcrenin sitozolunda meydana gelir Glukoz glukoz6


![The tense conformation of PFK, at high [ATP], has lower affinity for the other The tense conformation of PFK, at high [ATP], has lower affinity for the other](https://slidetodoc.com/presentation_image_h/3287c2575bb0bad4281a24fbc41ee13e/image-43.jpg)
![Inhibition of the Glycolysis enzyme Phosphofructokinase when [ATP] is high prevents breakdown of glucose Inhibition of the Glycolysis enzyme Phosphofructokinase when [ATP] is high prevents breakdown of glucose](https://slidetodoc.com/presentation_image_h/3287c2575bb0bad4281a24fbc41ee13e/image-44.jpg)
- Slides: 44

Glikoliz.

Glikoliz , hücrenin sitozolunda meydana gelir. Glukoz, glukoz-6 -fosfata dönüşerek glikoliz yoluna girer. Başlangıçta, ATP’nin iki ~P bağının kırılmasına bağlı olarak enerji girişi olur.

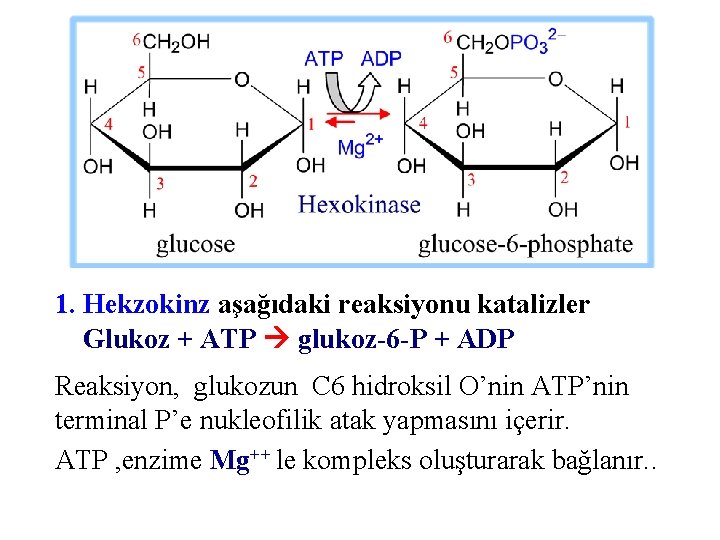
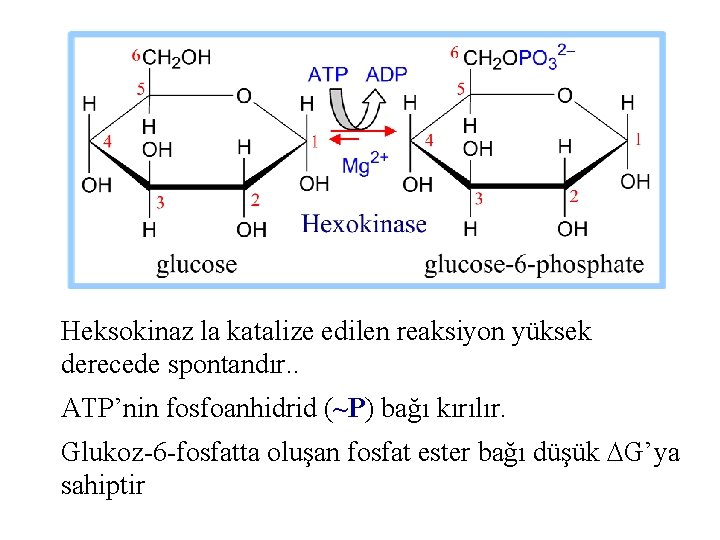
1. Hekzokinz aşağıdaki reaksiyonu katalizler Glukoz + ATP glukoz-6 -P + ADP Reaksiyon, glukozun C 6 hidroksil O’nin ATP’nin terminal P’e nukleofilik atak yapmasını içerir. ATP , enzime Mg++ le kompleks oluşturarak bağlanır. .

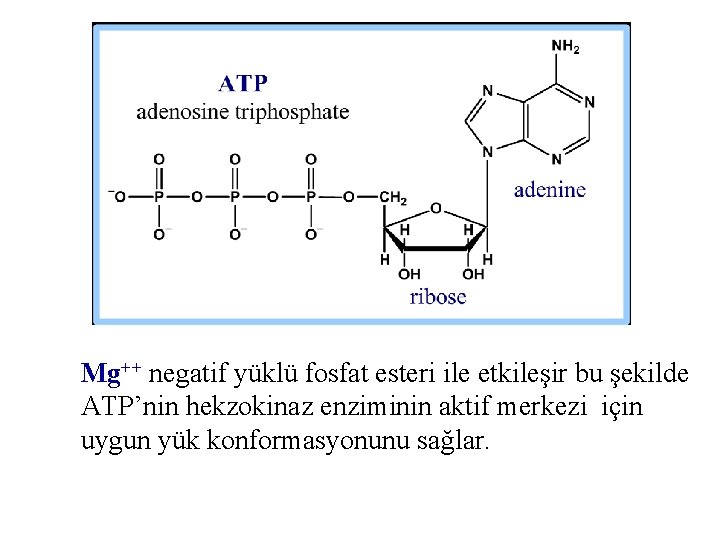
Mg++ negatif yüklü fosfat esteri ile etkileşir bu şekilde ATP’nin hekzokinaz enziminin aktif merkezi için uygun yük konformasyonunu sağlar.

Heksokinaz la katalize edilen reaksiyon yüksek derecede spontandır. . ATP’nin fosfoanhidrid (~P) bağı kırılır. Glukoz-6 -fosfatta oluşan fosfat ester bağı düşük DG’ya sahiptir

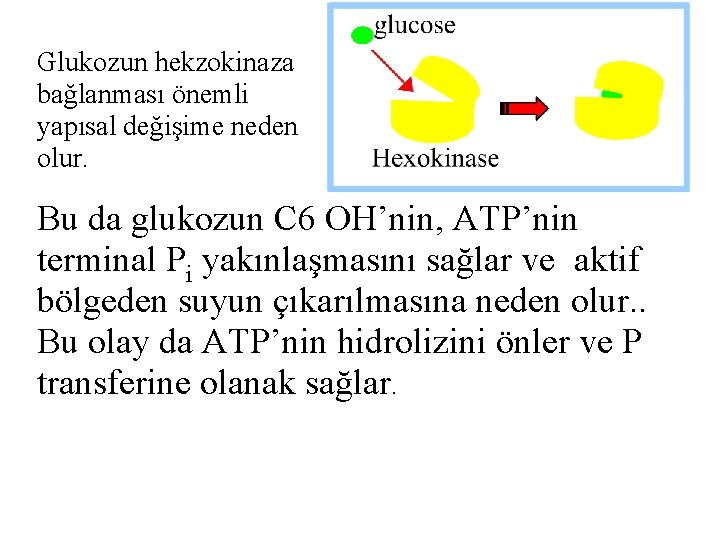
Glukozun hekzokinaza bağlanması önemli yapısal değişime neden olur. Bu da glukozun C 6 OH’nin, ATP’nin terminal Pi yakınlaşmasını sağlar ve aktif bölgeden suyun çıkarılmasına neden olur. . Bu olay da ATP’nin hidrolizini önler ve P transferine olanak sağlar.

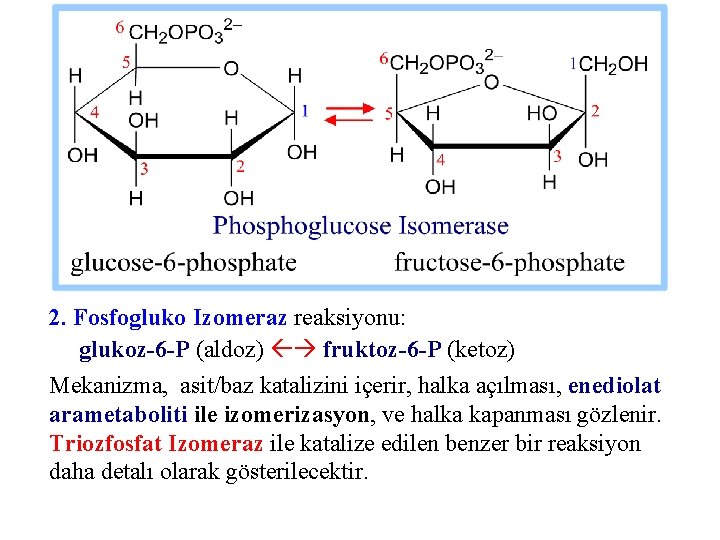
2. Fosfogluko Izomeraz reaksiyonu: glukoz-6 -P (aldoz) fruktoz-6 -P (ketoz) Mekanizma, asit/baz katalizini içerir, halka açılması, enediolat arametaboliti ile izomerizasyon, ve halka kapanması gözlenir. Triozfosfat Izomeraz ile katalize edilen benzer bir reaksiyon daha detalı olarak gösterilecektir.

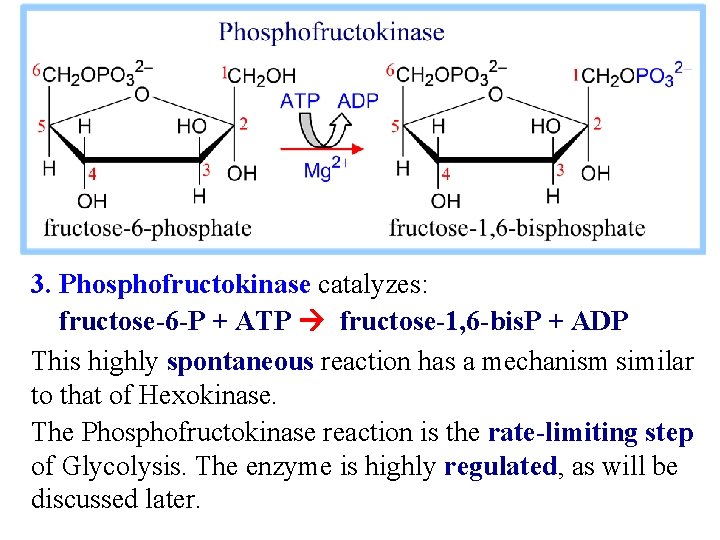
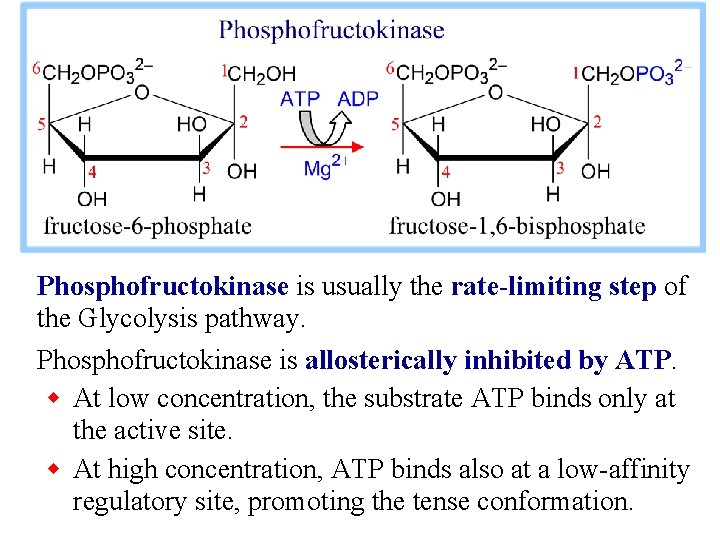
3. Phosphofructokinase catalyzes: fructose-6 -P + ATP fructose-1, 6 -bis. P + ADP This highly spontaneous reaction has a mechanism similar to that of Hexokinase. The Phosphofructokinase reaction is the rate-limiting step of Glycolysis. The enzyme is highly regulated, as will be discussed later.

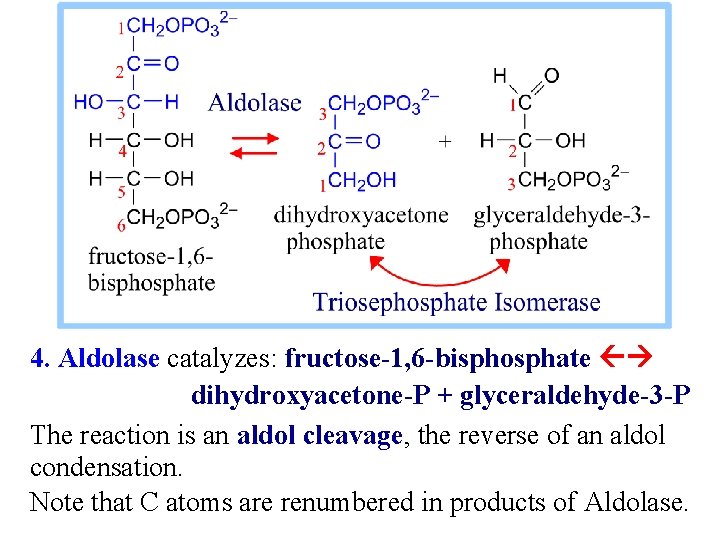
4. Aldolase catalyzes: fructose-1, 6 -bisphosphate dihydroxyacetone-P + glyceraldehyde-3 -P The reaction is an aldol cleavage, the reverse of an aldol condensation. Note that C atoms are renumbered in products of Aldolase.

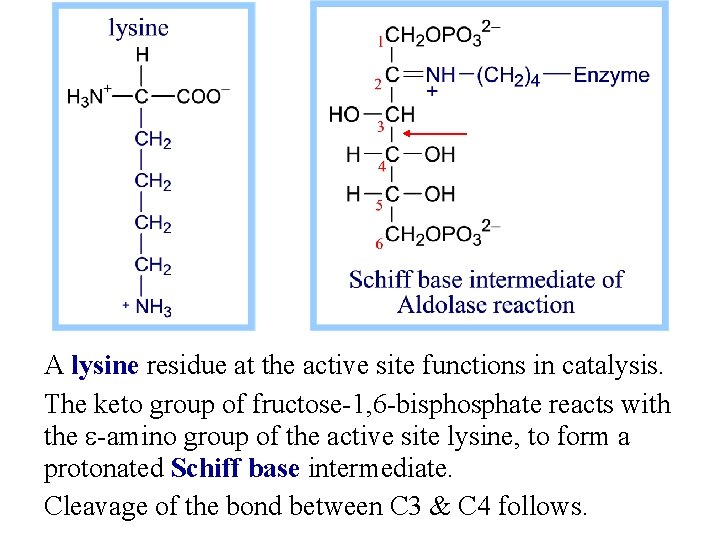
A lysine residue at the active site functions in catalysis. The keto group of fructose-1, 6 -bisphosphate reacts with the e-amino group of the active site lysine, to form a protonated Schiff base intermediate. Cleavage of the bond between C 3 & C 4 follows.

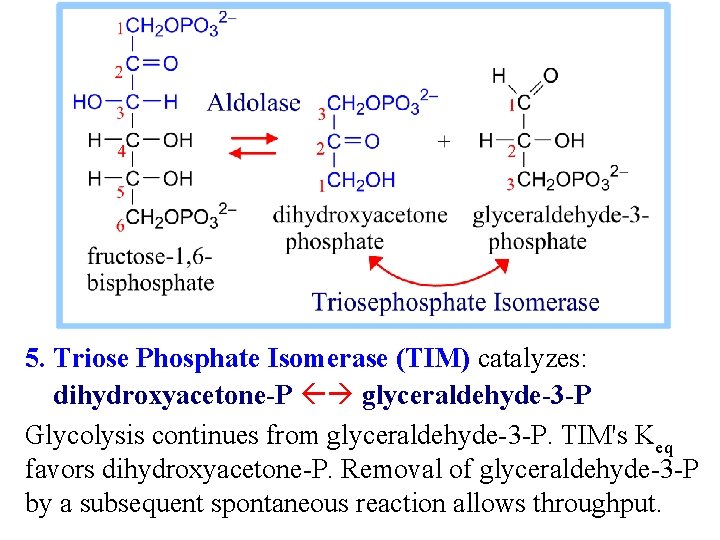
5. Triose Phosphate Isomerase (TIM) catalyzes: dihydroxyacetone-P glyceraldehyde-3 -P Glycolysis continues from glyceraldehyde-3 -P. TIM's Keq favors dihydroxyacetone-P. Removal of glyceraldehyde-3 -P by a subsequent spontaneous reaction allows throughput.

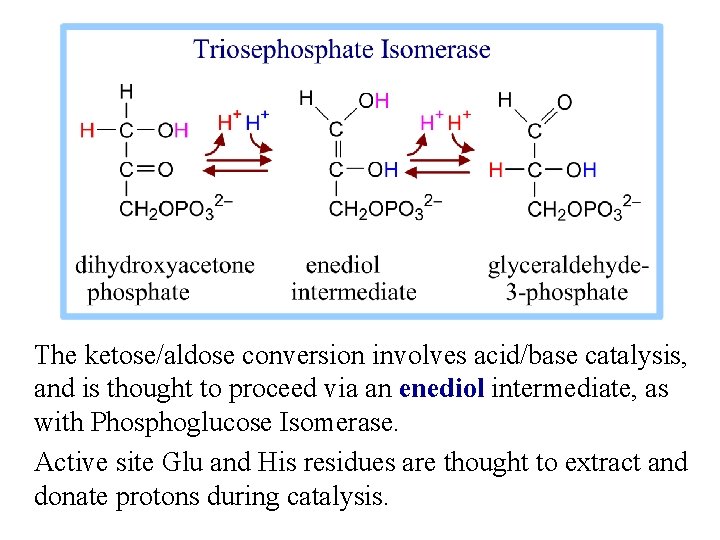
The ketose/aldose conversion involves acid/base catalysis, and is thought to proceed via an enediol intermediate, as with Phosphoglucose Isomerase. Active site Glu and His residues are thought to extract and donate protons during catalysis.

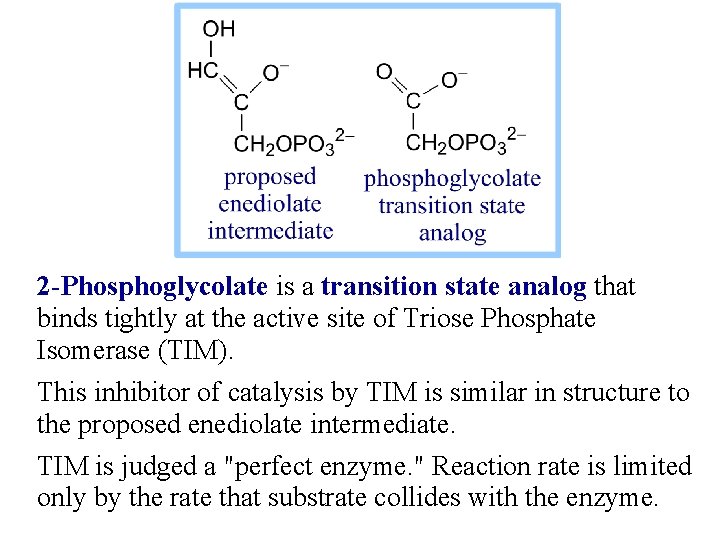
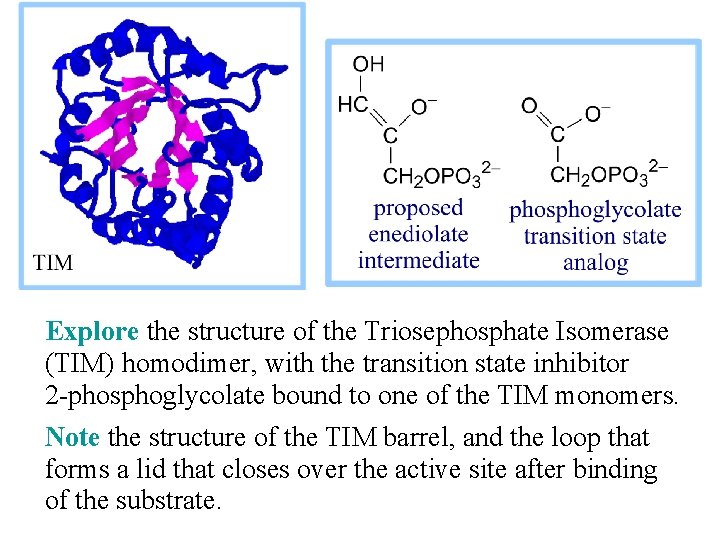
2 -Phosphoglycolate is a transition state analog that binds tightly at the active site of Triose Phosphate Isomerase (TIM). This inhibitor of catalysis by TIM is similar in structure to the proposed enediolate intermediate. TIM is judged a "perfect enzyme. " Reaction rate is limited only by the rate that substrate collides with the enzyme.

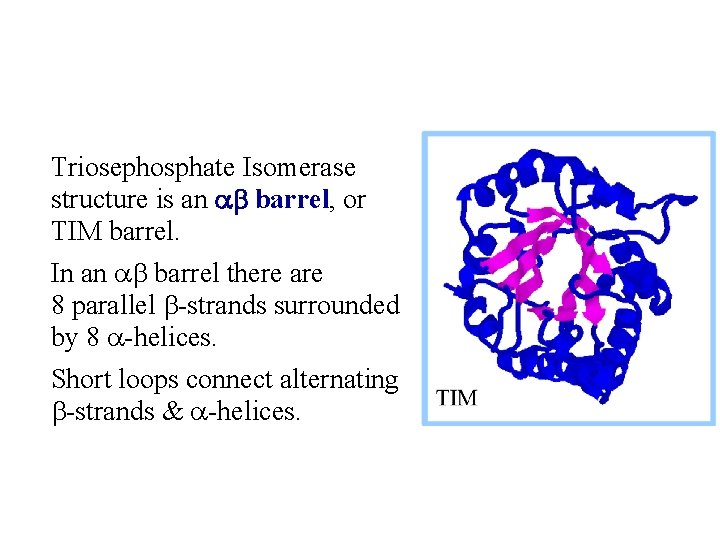
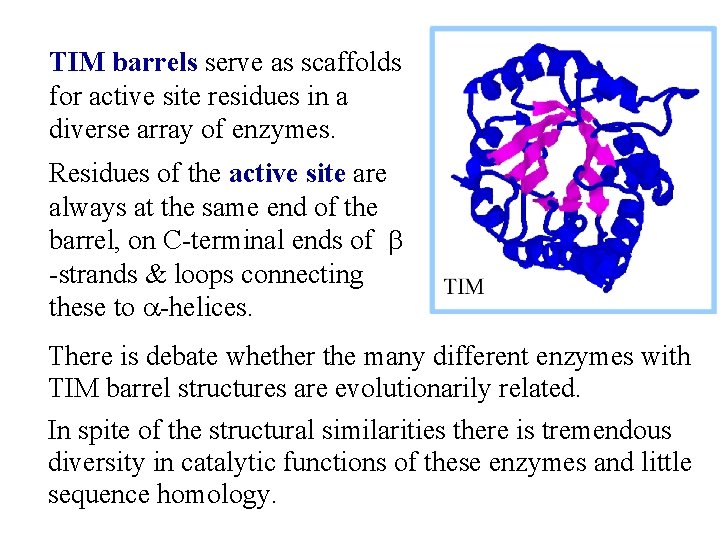
Triosephosphate Isomerase structure is an ab barrel, or TIM barrel. In an ab barrel there are 8 parallel b-strands surrounded by 8 a-helices. Short loops connect alternating b-strands & a-helices.

TIM barrels serve as scaffolds for active site residues in a diverse array of enzymes. Residues of the active site are always at the same end of the barrel, on C-terminal ends of b -strands & loops connecting these to a-helices. There is debate whether the many different enzymes with TIM barrel structures are evolutionarily related. In spite of the structural similarities there is tremendous diversity in catalytic functions of these enzymes and little sequence homology.

Explore the structure of the Triosephosphate Isomerase (TIM) homodimer, with the transition state inhibitor 2 -phosphoglycolate bound to one of the TIM monomers. Note the structure of the TIM barrel, and the loop that forms a lid that closes over the active site after binding of the substrate.

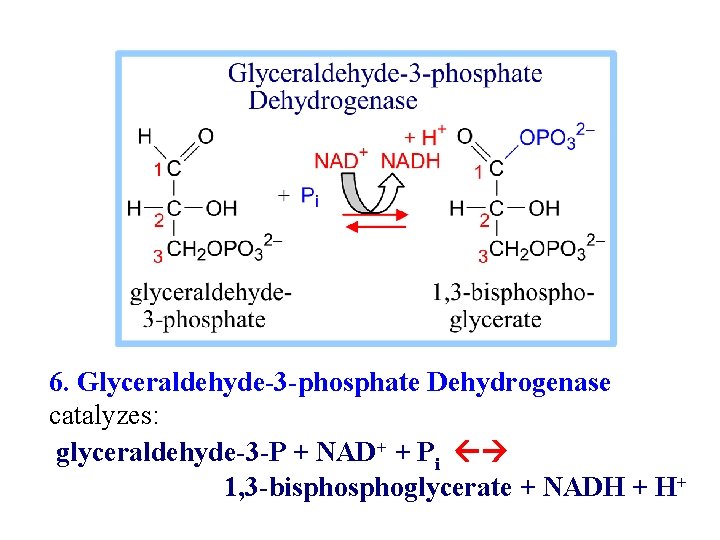
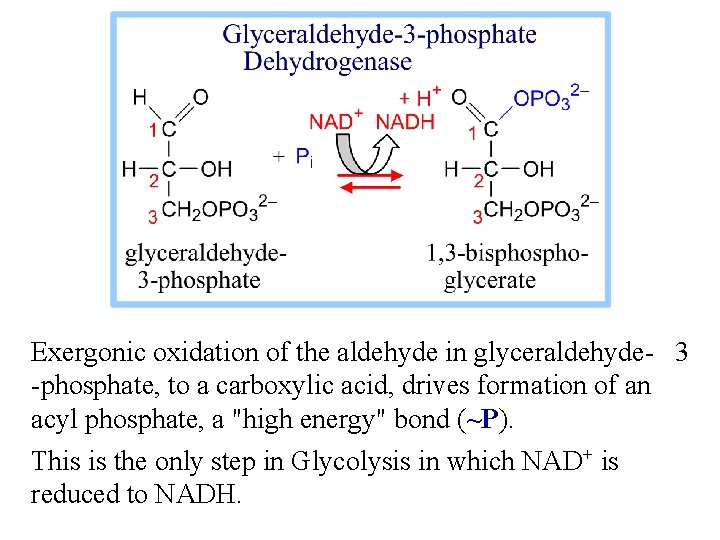
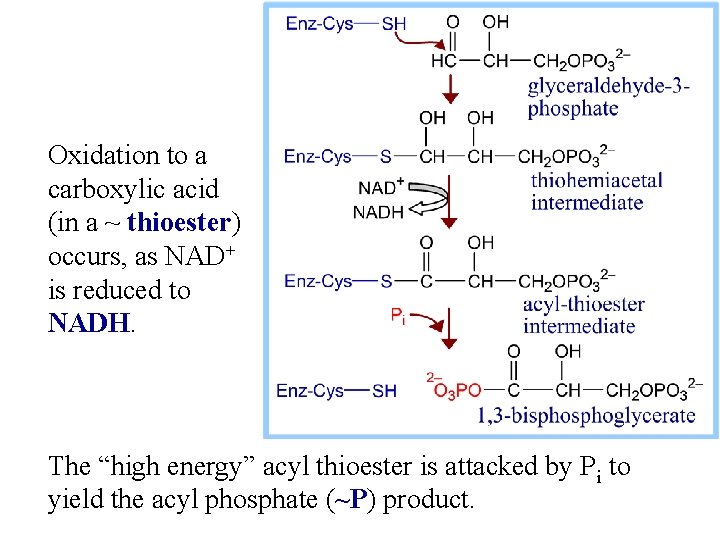
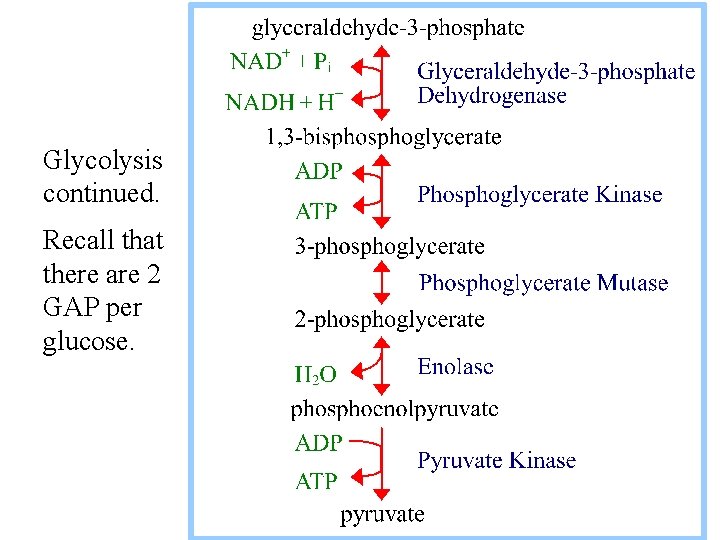
6. Glyceraldehyde-3 -phosphate Dehydrogenase catalyzes: glyceraldehyde-3 -P + NAD+ + Pi 1, 3 -bisphoglycerate + NADH + H+

Exergonic oxidation of the aldehyde in glyceraldehyde- 3 -phosphate, to a carboxylic acid, drives formation of an acyl phosphate, a "high energy" bond (~P). This is the only step in Glycolysis in which NAD+ is reduced to NADH.

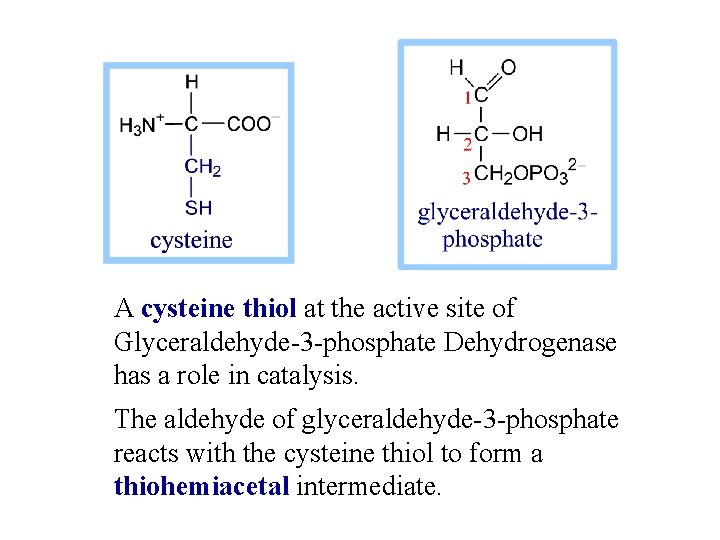
A cysteine thiol at the active site of Glyceraldehyde-3 -phosphate Dehydrogenase has a role in catalysis. The aldehyde of glyceraldehyde-3 -phosphate reacts with the cysteine thiol to form a thiohemiacetal intermediate.

Oxidation to a carboxylic acid (in a ~ thioester) occurs, as NAD+ is reduced to NADH. The “high energy” acyl thioester is attacked by Pi to yield the acyl phosphate (~P) product.

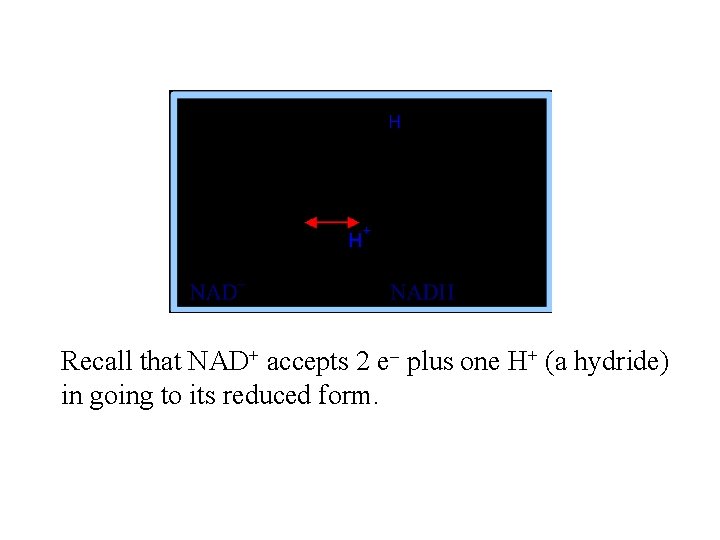
Recall that NAD+ accepts 2 e- plus one H+ (a hydride) in going to its reduced form.

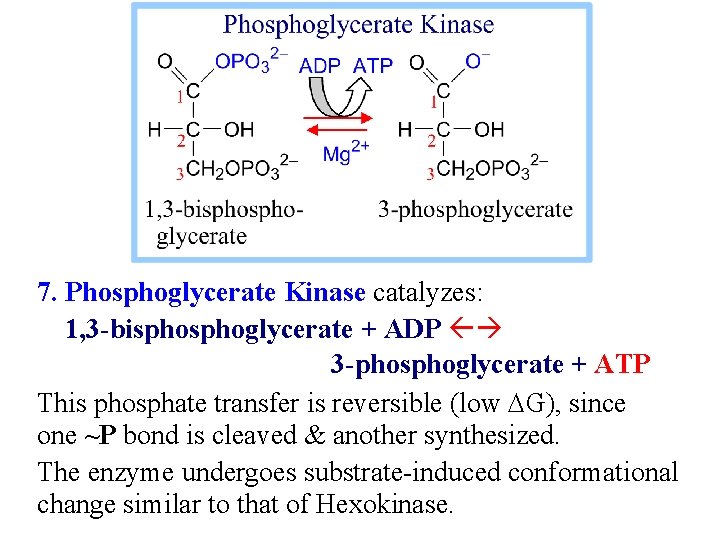
7. Phosphoglycerate Kinase catalyzes: 1, 3 -bisphoglycerate + ADP 3 -phosphoglycerate + ATP This phosphate transfer is reversible (low DG), since one ~P bond is cleaved & another synthesized. The enzyme undergoes substrate-induced conformational change similar to that of Hexokinase.

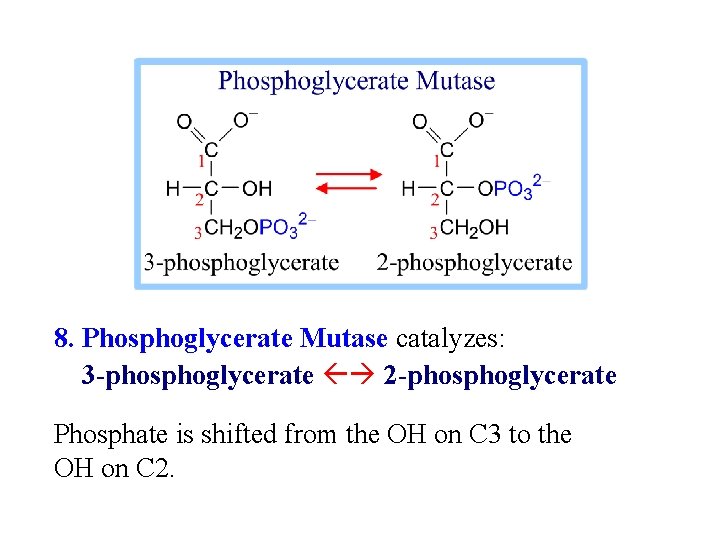
8. Phosphoglycerate Mutase catalyzes: 3 -phosphoglycerate 2 -phosphoglycerate Phosphate is shifted from the OH on C 3 to the OH on C 2.

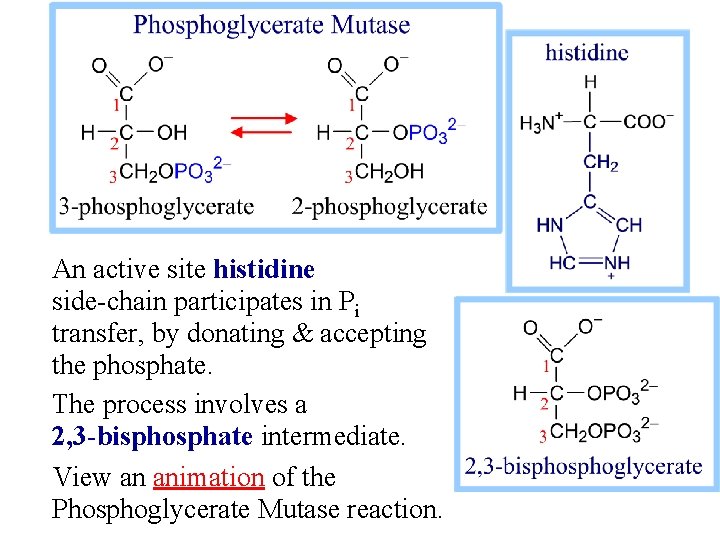
An active site histidine side-chain participates in Pi transfer, by donating & accepting the phosphate. The process involves a 2, 3 -bisphosphate intermediate. View an animation of the Phosphoglycerate Mutase reaction.

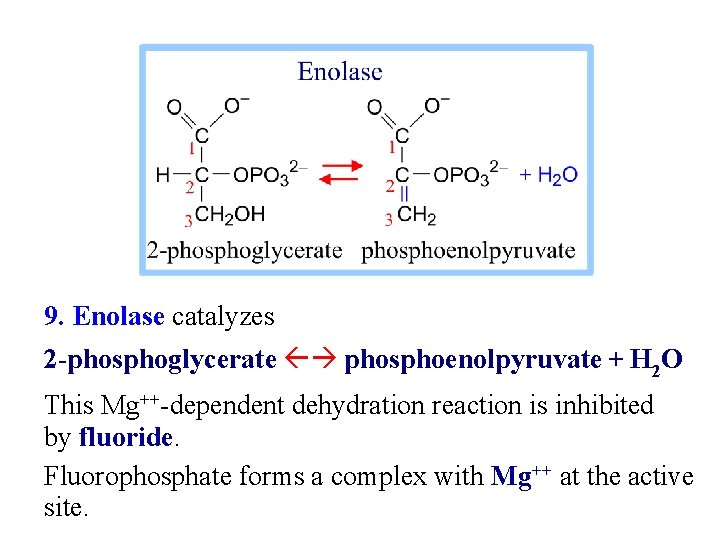
9. Enolase catalyzes 2 -phosphoglycerate phosphoenolpyruvate + H 2 O This Mg++-dependent dehydration reaction is inhibited by fluoride. Fluorophosphate forms a complex with Mg++ at the active site.

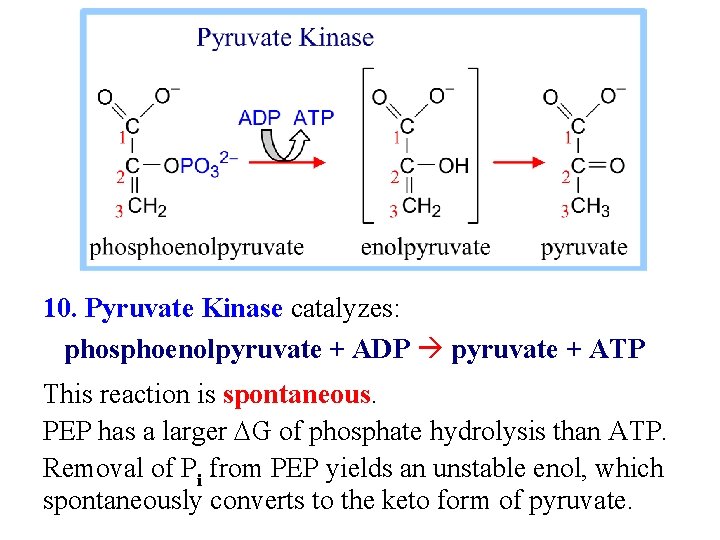
10. Pyruvate Kinase catalyzes: phosphoenolpyruvate + ADP pyruvate + ATP This reaction is spontaneous. PEP has a larger DG of phosphate hydrolysis than ATP. Removal of Pi from PEP yields an unstable enol, which spontaneously converts to the keto form of pyruvate.


Glycolysis continued. Recall that there are 2 GAP per glucose.

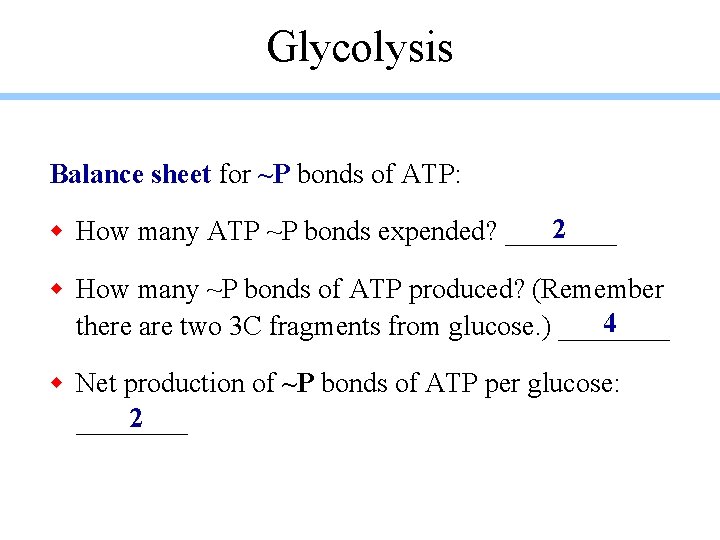
Glycolysis Balance sheet for ~P bonds of ATP: 2 w How many ATP ~P bonds expended? ____ w How many ~P bonds of ATP produced? (Remember 4 there are two 3 C fragments from glucose. ) ____ w Net production of ~P bonds of ATP per glucose: 2 ____

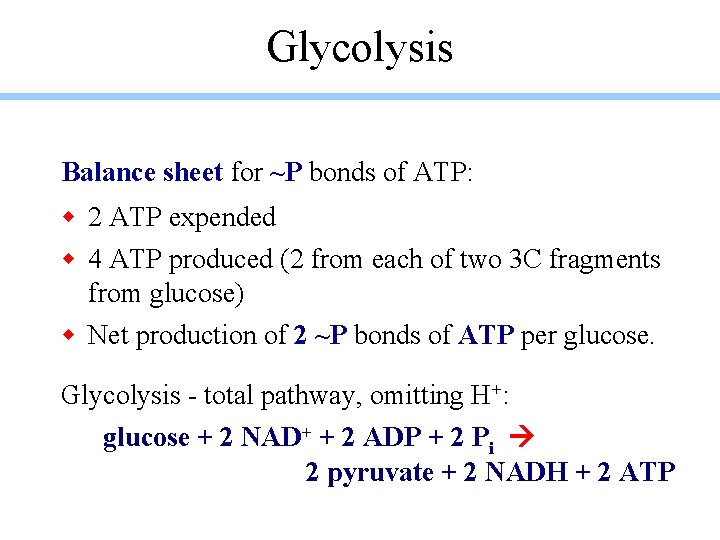
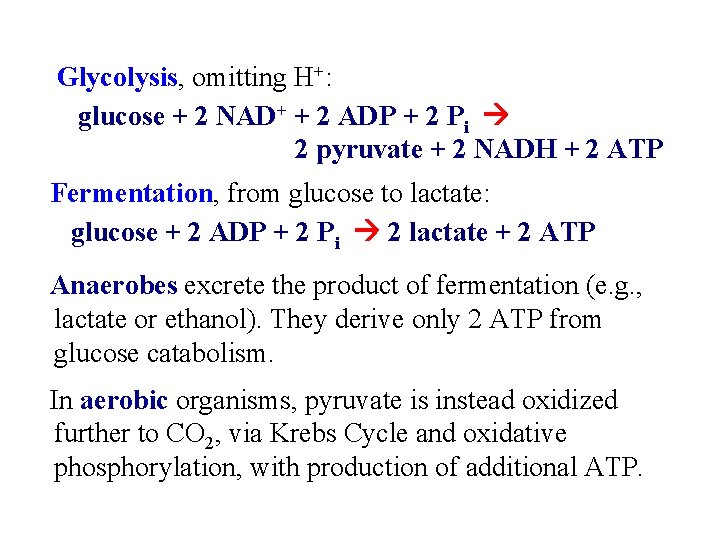
Glycolysis Balance sheet for ~P bonds of ATP: w 2 ATP expended w 4 ATP produced (2 from each of two 3 C fragments from glucose) w Net production of 2 ~P bonds of ATP per glucose. Glycolysis - total pathway, omitting H+: glucose + 2 NAD+ + 2 ADP + 2 Pi 2 pyruvate + 2 NADH + 2 ATP

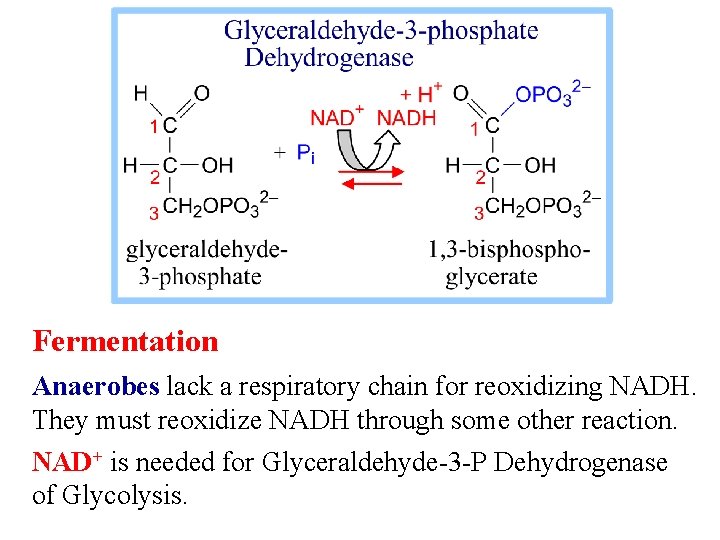
Fermentation Anaerobes lack a respiratory chain for reoxidizing NADH. They must reoxidize NADH through some other reaction. NAD+ is needed for Glyceraldehyde-3 -P Dehydrogenase of Glycolysis.

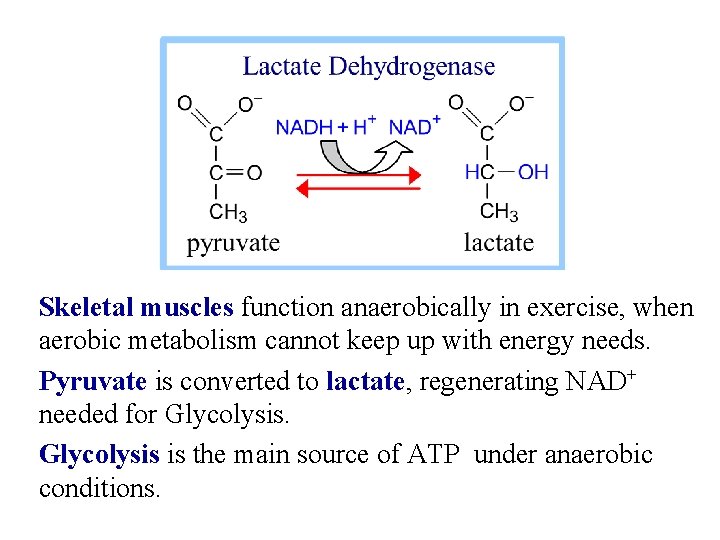
Skeletal muscles function anaerobically in exercise, when aerobic metabolism cannot keep up with energy needs. Pyruvate is converted to lactate, regenerating NAD+ needed for Glycolysis is the main source of ATP under anaerobic conditions.

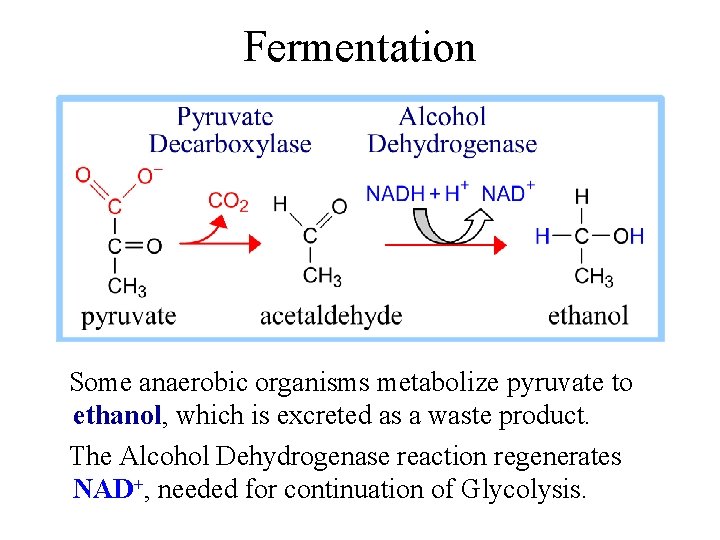
Fermentation Some anaerobic organisms metabolize pyruvate to ethanol, which is excreted as a waste product. The Alcohol Dehydrogenase reaction regenerates NAD+, needed for continuation of Glycolysis.

Glycolysis, omitting H+: glucose + 2 NAD+ + 2 ADP + 2 Pi 2 pyruvate + 2 NADH + 2 ATP Fermentation, from glucose to lactate: glucose + 2 ADP + 2 Pi 2 lactate + 2 ATP Anaerobes excrete the product of fermentation (e. g. , lactate or ethanol). They derive only 2 ATP from glucose catabolism. In aerobic organisms, pyruvate is instead oxidized further to CO 2, via Krebs Cycle and oxidative phosphorylation, with production of additional ATP.

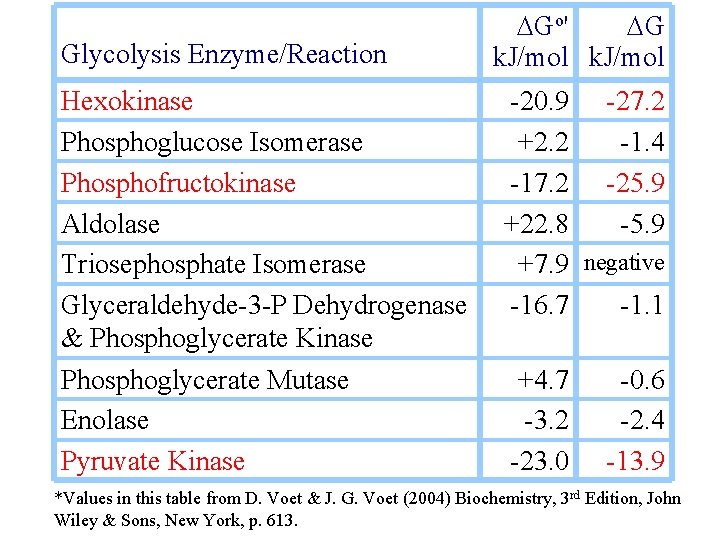
Glycolysis Enzyme/Reaction DGo' DG k. J/mol Hexokinase Phosphoglucose Isomerase Phosphofructokinase Aldolase Triosephosphate Isomerase Glyceraldehyde-3 -P Dehydrogenase & Phosphoglycerate Kinase -20. 9 -27. 2 +2. 2 -1. 4 -17. 2 -25. 9 +22. 8 -5. 9 +7. 9 negative -16. 7 -1. 1 Phosphoglycerate Mutase Enolase Pyruvate Kinase +4. 7 -3. 2 -23. 0 -0. 6 -2. 4 -13. 9 *Values in this table from D. Voet & J. G. Voet (2004) Biochemistry, 3 rd Edition, John Wiley & Sons, New York, p. 613.

Three Glycolysis enzymes catalyze spontaneous reactions: Hexokinase, Phosphofructokinase & Pyruvate Kinase. Control of these enzymes determines the rate of the Glycolysis pathway. Local control involves dependence of enzyme-catalyzed reactions on concentrations of pathway substrates or intermediates within a cell. Global control involves hormone-activated production of second messengers that regulate cellular reactions for the benefit of the organism as a whole. Local control will be discussed here. Regulation by hormone-activated c. AMP signal cascade will be discussed later.

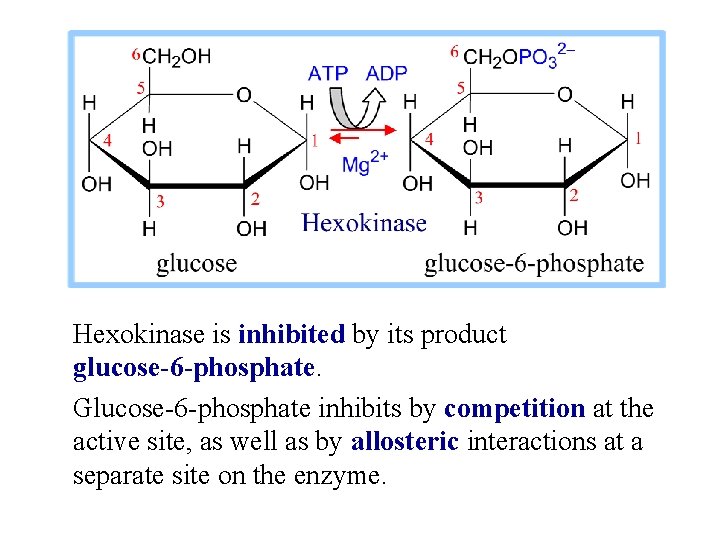
Hexokinase is inhibited by its product glucose-6 -phosphate. Glucose-6 -phosphate inhibits by competition at the active site, as well as by allosteric interactions at a separate site on the enzyme.

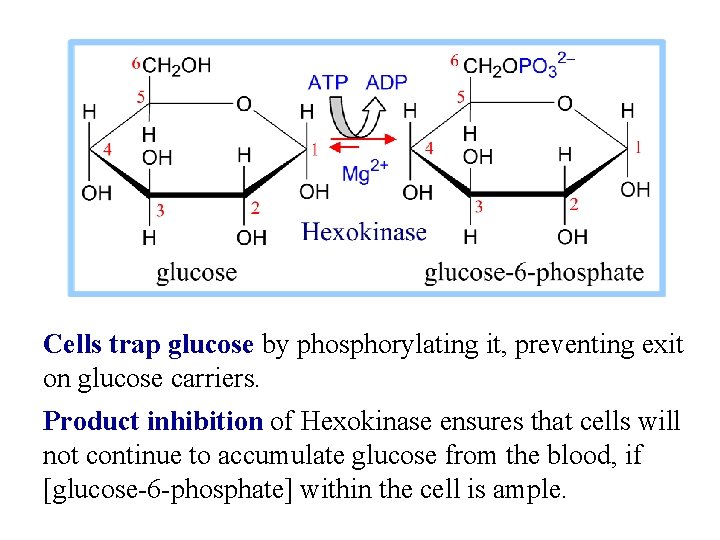
Cells trap glucose by phosphorylating it, preventing exit on glucose carriers. Product inhibition of Hexokinase ensures that cells will not continue to accumulate glucose from the blood, if [glucose-6 -phosphate] within the cell is ample.

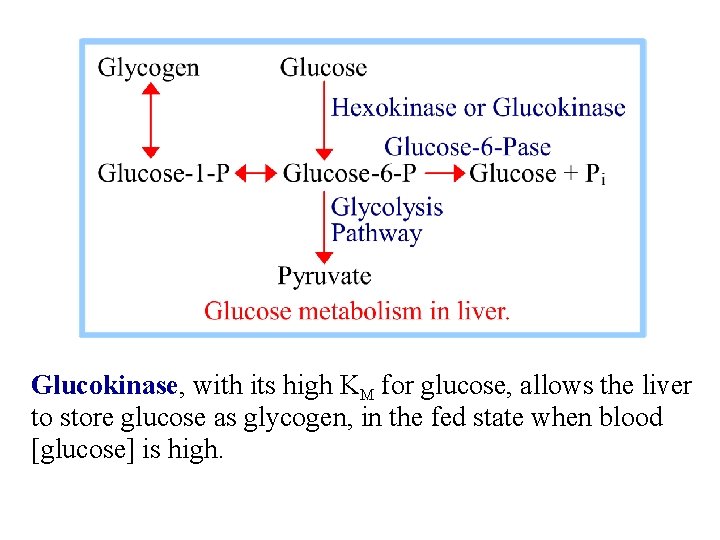
Glucokinase, a variant of Hexokinase found in liver, has a high KM for glucose. It is active only at high [glucose]. Glucokinase is not subject to product inhibition by glucose-6 -phosphate. Liver will take up & phosphorylate glucose even when liver [glucose-6 -phosphate] is high. Liver Glucokinase is subject to inhibition by glucokinase regulatory protein (GKRP). The ratio of Glucokinase to GKRP changes in different metabolic states, providing a mechanism for modulating glucose phosphorylation.

Glucokinase, with its high KM for glucose, allows the liver to store glucose as glycogen, in the fed state when blood [glucose] is high.

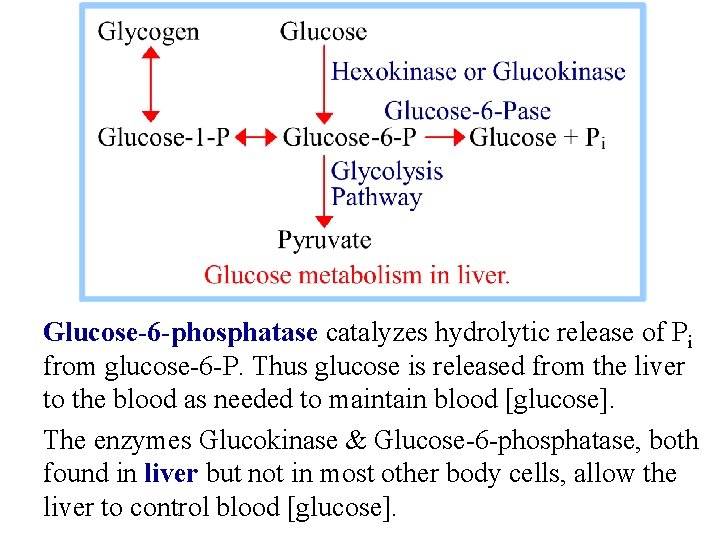
Glucose-6 -phosphatase catalyzes hydrolytic release of Pi from glucose-6 -P. Thus glucose is released from the liver to the blood as needed to maintain blood [glucose]. The enzymes Glucokinase & Glucose-6 -phosphatase, both found in liver but not in most other body cells, allow the liver to control blood [glucose].

Phosphofructokinase is usually the rate-limiting step of the Glycolysis pathway. Phosphofructokinase is allosterically inhibited by ATP. w At low concentration, the substrate ATP binds only at the active site. w At high concentration, ATP binds also at a low-affinity regulatory site, promoting the tense conformation.
![The tense conformation of PFK at high ATP has lower affinity for the other The tense conformation of PFK, at high [ATP], has lower affinity for the other](https://slidetodoc.com/presentation_image_h/3287c2575bb0bad4281a24fbc41ee13e/image-43.jpg)
The tense conformation of PFK, at high [ATP], has lower affinity for the other substrate, fructose-6 -P. Sigmoidal dependence of reaction rate on [fructose-6 -P] is seen. AMP, present at significant levels only when there is extensive ATP hydrolysis, antagonizes effects of high ATP.
![Inhibition of the Glycolysis enzyme Phosphofructokinase when ATP is high prevents breakdown of glucose Inhibition of the Glycolysis enzyme Phosphofructokinase when [ATP] is high prevents breakdown of glucose](https://slidetodoc.com/presentation_image_h/3287c2575bb0bad4281a24fbc41ee13e/image-44.jpg)
Inhibition of the Glycolysis enzyme Phosphofructokinase when [ATP] is high prevents breakdown of glucose in a pathway whose main role is to make ATP. It is more useful to the cell to store glucose as glycogen when ATP is plentiful.
 Antropozoik zaman
Antropozoik zaman Imalatçı aleyhine matrahta meydana gelen değişiklik
Imalatçı aleyhine matrahta meydana gelen değişiklik Yeryüzüne yakın yerlerde gerçekleşen hava olayları
Yeryüzüne yakın yerlerde gerçekleşen hava olayları Yapılan ya da olan iş meydana gelen şey
Yapılan ya da olan iş meydana gelen şey Karboksilaz enzimi
Karboksilaz enzimi F tipi atpaz
F tipi atpaz Kandan glukoz alan olaylar
Kandan glukoz alan olaylar çölyak çomak parmak
çölyak çomak parmak Glikoliz glukoneogenez
Glikoliz glukoneogenez Mutarotasyon
Mutarotasyon Fehling tollens tepkimeleri
Fehling tollens tepkimeleri Gliserol glukoneogenez
Gliserol glukoneogenez Karbonhidratların sınıflandırılması
Karbonhidratların sınıflandırılması Glukoz oksidaz metodu
Glukoz oksidaz metodu Na glukoz simportu
Na glukoz simportu Kanda glukoz tayini
Kanda glukoz tayini Mannoz oluşumu
Mannoz oluşumu Glukozaning parchalanishi
Glukozaning parchalanishi Glikoliz ve glukoneogenez ortak enzim
Glikoliz ve glukoneogenez ortak enzim Pentoz fosfat yolağı
Pentoz fosfat yolağı Fosfoglukomutaz
Fosfoglukomutaz Eritrositlerde glikolizin yan ürünü
Eritrositlerde glikolizin yan ürünü Enediolate
Enediolate Milli gelir nedir
Milli gelir nedir Gelir tablosu
Gelir tablosu Gelir tablosu ilkeleri
Gelir tablosu ilkeleri Pazarlama aktivite planı
Pazarlama aktivite planı Gelir ve gider hesapları
Gelir ve gider hesapları Altıgenin alanı
Altıgenin alanı Marjinal tüketim eğilimi
Marjinal tüketim eğilimi Geçici işaretler
Geçici işaretler Meram tip kan istem formu
Meram tip kan istem formu Sürekli gelir hipotezi nedir
Sürekli gelir hipotezi nedir Hipotez örnekleri
Hipotez örnekleri Gelir modeli örnekleri
Gelir modeli örnekleri Ne anlama gelir
Ne anlama gelir Havadan gelir top gibi suda erir hap gibi
Havadan gelir top gibi suda erir hap gibi Proforma gelir tablosu nedir
Proforma gelir tablosu nedir Optimal piyasa nedir
Optimal piyasa nedir Kamu iktisadi teşebbüsleri
Kamu iktisadi teşebbüsleri Tdhp gelir tablosu
Tdhp gelir tablosu 1479-4/b-1 gelir vergisi mükellefi
1479-4/b-1 gelir vergisi mükellefi Yer yön bildiren sözcüklerin yazımı
Yer yön bildiren sözcüklerin yazımı Juan motosikleti ile meksika sınırına gelir
Juan motosikleti ile meksika sınırına gelir Szsz
Szsz